20.2 Outline a procedure for separating a mixture
20.2 Outline a procedure for separating a mixture
20.2 Outline a procedure for separating a mixture
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
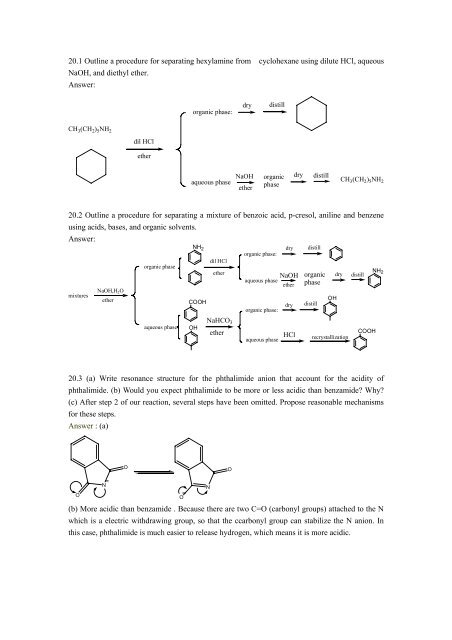
20.1 <strong>Outline</strong> a <strong>procedure</strong> <strong>for</strong> <strong>separating</strong> hexylamine from cyclohexane using dilute HCl, aqueous<br />
NaOH, and diethyl ether.<br />
Answer:<br />
CH 3(CH 2) 5NH 2<br />
dil HCl<br />
ether<br />
organic phase:<br />
aqueous phase<br />
dry distill<br />
NaOH<br />
ether<br />
organic<br />
phase<br />
dry distill<br />
CH 3(CH 2) 5NH 2<br />
<strong>20.2</strong> <strong>Outline</strong> a <strong>procedure</strong> <strong>for</strong> <strong>separating</strong> a <strong>mixture</strong> of benzoic acid, p-cresol, aniline and benzene<br />
using acids, bases, and organic solvents.<br />
Answer:<br />
<strong>mixture</strong>s<br />
organic phase<br />
NH 2<br />
NaOH,H2O ether COOH<br />
aqueous phase<br />
OH<br />
dil HCl<br />
ether<br />
NaHCO 3<br />
ether<br />
organic phase:<br />
NaOH<br />
aqueous phase<br />
ether<br />
organic phase:<br />
aqueous phase<br />
dry distill<br />
dry<br />
HCl<br />
organic<br />
phase<br />
distill<br />
OH<br />
recrystallization<br />
dry distill<br />
COOH<br />
20.3 (a) Write resonance structure <strong>for</strong> the phthalimide anion that account <strong>for</strong> the acidity of<br />
phthalimide. (b) Would you expect phthalimide to be more or less acidic than benzamide? Why?<br />
(c) After step 2 of our reaction, several steps have been omitted. Propose reasonable mechanisms<br />
<strong>for</strong> these steps.<br />
Answer : (a)<br />
O<br />
N<br />
O<br />
O<br />
N<br />
(b) More acidic than benzamide . Because there are two C=O (carbonyl groups) attached to the N<br />
which is a electric withdrawing group, so that the ccarbonyl group can stabilize the N anion. In<br />
this case, phthalimide is much easier to release hydrogen, which means it is more acidic.<br />
O<br />
NH 2
(c)<br />
O<br />
O<br />
N R H 2N NH 2<br />
O<br />
NH<br />
NH<br />
O HN R<br />
O<br />
O<br />
H<br />
N NH 2<br />
N R<br />
20.4 <strong>Outline</strong> a preparation of benzylamine using the Gabriel synthesis.<br />
O<br />
N<br />
H<br />
O<br />
KOH X<br />
O<br />
O<br />
OH<br />
ethanol<br />
N<br />
H<br />
H<br />
N<br />
NH2 several steps<br />
O<br />
K<br />
N<br />
H<br />
H<br />
O<br />
O<br />
N<br />
N<br />
O<br />
O<br />
H<br />
H<br />
NH<br />
NH<br />
O<br />
O<br />
+<br />
N<br />
+ H 2N<br />
O<br />
O<br />
H 2N R<br />
H 2N NH 2<br />
20.5 Show how you might prepare each of the following amines through reductive amination:<br />
a) CH3(CH2)3CH2NH2<br />
H<br />
NH3,H2,Ni O<br />
b) C6H5CH2CH(NH2)CH3<br />
O<br />
c) CH3(CH2)4CH2NHC6H5<br />
d) C6H5CH2N(CH3)2<br />
O<br />
H<br />
NH 3,H 2,Ni<br />
H 2,Ni<br />
NH 2<br />
NH 2<br />
N<br />
H<br />
N<br />
H<br />
N<br />
H<br />
NH 2<br />
NH 2<br />
R
O<br />
H<br />
H<br />
H 2,Ni<br />
N<br />
20.6 Reductive amination of a ketone is almost always a better method <strong>for</strong> the preparation of<br />
amines of the type RCH(R’)NH2 than treatment of an alkyl halide with ammonia. Why would<br />
this be true?<br />
Answer:<br />
I think the reasons are: 1) The multiple alkylations occurs when alkyl halide reacts with<br />
ammonium, so the method is of very limited synthetic application. 2) I found that if it is treated by<br />
ketone, the possible way is only one, so this reaction can be controlled well.<br />
Above all, we can reach the conclusion easily that reductive amination of a ketone is almost<br />
always a better method <strong>for</strong> the preparation of amines.<br />
20.7 Show how you might utilize the reduction of an amide, oxime, or nitrile to carry out each of<br />
the following trans<strong>for</strong>mations:<br />
(a) Benzoic acid to benzylethylamine<br />
(b) 1-Bromopentane to hexylamine<br />
(c) Propanoic acid to tripropylamine<br />
(d) Butanone to sec-butylamine<br />
Answer:<br />
(a)<br />
(b)<br />
(c) CH 3CH 2COOH<br />
(d)<br />
COOH COCl COHN<br />
SOCl 2<br />
NH 2C 2H 5<br />
NaCN<br />
CH2Br(CH2)3CH3 CH2CN(CH2)3CH3<br />
SOCl 2<br />
O<br />
O<br />
Cl<br />
NH3<br />
LiBH3CN NH(CH 2CH 2CH 3) 2<br />
N<br />
NH 2<br />
O<br />
(1) LiALH 4,Et 2O<br />
(2) H 2O<br />
(1) LiALH 4,Et 2O<br />
(2) H 2O<br />
N(CH 2CH 2CH 3) 2<br />
(1) LiALH 4,Et 2O<br />
(2) H 2O<br />
H<br />
N<br />
NH 2(CH 2) 5CH 3<br />
(CH 3CH 2CH 2) 3N
20.8 Using a different method <strong>for</strong> each part, but taking care in each case to select a good method,<br />
show how each of the following trans<strong>for</strong>mations might be accomplished:<br />
(a)<br />
(b)<br />
(c)<br />
(d)<br />
(e)<br />
Answer:<br />
(a)<br />
H 3CO<br />
H 3CO<br />
H 3CO H 3CO CHNH 2CH 3<br />
CH 3<br />
NH 2<br />
CH 2N(CH 3) 3Cl<br />
O2N CH3 O2N NH2 HNO3 H3CO H3CO H2SO4 (b) H 3CO<br />
(c) Cl 2/hv<br />
(d)<br />
O2N CH3<br />
CH 3 CH 2CH 2NH 2<br />
NO 2<br />
(1) Fe,HCl<br />
(2) OH<br />
H 3CO NH 2<br />
AlCl3 CH3COCl H3CO<br />
O<br />
CCH3<br />
NH3 NaBH3CN H3CO CHNH2CH3 CH 2Cl<br />
N(CH 3) 3<br />
T.M<br />
KMnO SOCl<br />
4,H 2<br />
O2N COOH O2N COCl<br />
(1)NaN 3 O2N NH 2<br />
(2)H2O
(e) CH 3<br />
NBS/hv<br />
CH 2Br NaCN<br />
(i) LiAlH 4<br />
CH 2CN (ii) H2O<br />
20.9 Review the chemistry of amines given in earlier sections and provide a specific example <strong>for</strong><br />
each of the previously illustrated reactions.<br />
Answer:<br />
P963 The Hofmann Rearrangement<br />
O<br />
NH 2<br />
P960 Reductive Amination<br />
O<br />
+ Br 2 + 4NaOH<br />
+ NH 2<br />
H 2O<br />
H +<br />
NH 2<br />
CH 2CH 2NH 2<br />
+ Na2CO3 + 2NaBr + 2H2O 20.10 Para-nitrosation of N,N-dimethylaniline (C-nitrosation) is believed to take place through an<br />
electrophilic attack by NO + ions.<br />
(a) Show how NO + ions might be <strong>for</strong>med in an aqueous solution of NaNO2 and HCl.<br />
Answer:<br />
O N O<br />
H + H +<br />
O N OH O N OH 2 O N<br />
(b) Write a mechanism <strong>for</strong> p-nitrosation of N,N-demethylaniline.<br />
Answer:<br />
H2O N H<br />
O N<br />
N<br />
(c) Tertiary aromatic amines and phenols undergo C-nitrosation reaction, whereas most other<br />
benzene derivatives do not. How can you account <strong>for</strong> this difference?<br />
Answer:<br />
That is because nitrosonium cation is a kind of weak electrophile.<br />
H<br />
NO<br />
N<br />
N<br />
H<br />
NO
20.11 In the preceding examples of diazonium reactions, we have illustrated syntheses<br />
beginning with the compounds (a)-(e) here. Show how you might prepare each<br />
of the following compounds from benzene.<br />
(a) m-Nitroaniline (c) m-Bromoaniline (e) p-Nitroaniline<br />
(b) m-Chloroaniline (d) o-Nitroaniline<br />
Solution:<br />
(a)<br />
(b)<br />
(c)<br />
(d)<br />
(e)<br />
HNO 3,H 2SO 4<br />
HNO 3,H 2SO 4<br />
HNO 3,H 2SO 4<br />
HNO 3,H 2SO 4<br />
NHCOCH3<br />
SO3H<br />
HNO 3,H 2SO 4<br />
NO2<br />
NO 2<br />
NO2<br />
NO2<br />
HNO 3,H 2SO 4<br />
NO2<br />
HNO 3,H 2SO 4<br />
Cl 2,Fe<br />
Br 2,Fe<br />
1) Fe,HCl<br />
2) OH<br />
1) Fe,HCl<br />
2) OH<br />
NO 2<br />
NO 2<br />
NO2<br />
NH2<br />
NHCOCH 3<br />
SO 3H<br />
NH2<br />
Br<br />
H2S NH3,C2H5OH NO 2<br />
Cl<br />
1) Fe,HCl<br />
2) OH<br />
1) Fe,HCl<br />
2) OH<br />
CH3COCl<br />
NO2<br />
1) dilute,H2SO4 NHCOCH3<br />
NO2<br />
2) OH -<br />
CH 3COCl<br />
1) dilute H 2SO 4<br />
2) OH -<br />
NH 2<br />
NH 2<br />
NH 2<br />
NHCOCH 3<br />
NH 2<br />
NHCOCH 3<br />
NH 2<br />
NO 2<br />
Br<br />
Cl<br />
NO 2<br />
concd H 2SO 4<br />
NO 2<br />
HNO 3,H 2SO 4
20.12 Suggest how you might modify the preceding synthesis in order to prepare<br />
3.5-dibromotolune.<br />
Solution:<br />
CH 3<br />
NH 2<br />
Br 2,Fe<br />
Br<br />
CH3<br />
NH 2<br />
H 2SO 4,HNO 2<br />
Br<br />
Br<br />
CH 3<br />
N 2<br />
H 3PO 2,H 2O<br />
20.13 (a) In Section 20.8D we showed a synthesis of m-fluorotoluene starting with m-toluidine.<br />
How would you prepare m-toluidine from toluene? (b) How would you prepare m-chlorotoluene?<br />
(c) m-Bromotoluene? (d) m-Iodotoluene? (e) m-Tolunitrile (m-CH3C6H4CN)? (f) m-Toluic acid?<br />
Answer:<br />
(a)<br />
NHCOCH 3<br />
NO 2<br />
H 2SO 4<br />
HNO 3<br />
H 3O +<br />
NO 2<br />
Fe<br />
HCl<br />
NO 2<br />
NH 2<br />
Fe<br />
HCl<br />
NO 2<br />
NaNO 2<br />
HCl<br />
(CH 3CO) 2O<br />
Br<br />
Br<br />
NH 2 NHCOCH3<br />
NH 2<br />
N +<br />
NCl -<br />
NO 2<br />
H3PO2 C2H5OH CH 3<br />
H 2SO 4<br />
HNO 3<br />
Br
(b)<br />
(c)<br />
NHCOCH 3<br />
NHCOCH 3<br />
Cl<br />
Br<br />
H 2SO 4<br />
HNO 3<br />
H 3O +<br />
Cl<br />
H 2SO 4<br />
HNO 3<br />
H 3O +<br />
Br<br />
NO2<br />
NO2<br />
NH 2<br />
NH 2<br />
Fe<br />
HCl<br />
NaNO 2<br />
HCl<br />
Cl<br />
Fe<br />
HCl<br />
NaNO 2<br />
HCl<br />
Br<br />
(CH 3CO) 2O<br />
NH2 NHCOCH 3<br />
N +<br />
NCl -<br />
Cl<br />
(CH 3CO) 2O<br />
H3PO2 C2H5OH (1) Cl 2<br />
NH 2 NHCOCH3<br />
N +<br />
NCl -<br />
Br<br />
(2) OH - , H 2O<br />
heat<br />
H3PO2 C2H5OH (1) Br 2<br />
(2) OH - , H 2O<br />
heat
(d)<br />
(e)<br />
NHCOCH 3<br />
NHCOCH 3<br />
NHCOCH 3<br />
I<br />
CN<br />
NO2<br />
H 2SO 4<br />
HNO 3<br />
I<br />
H 3O +<br />
H 2SO 4<br />
HNO 3<br />
Fe<br />
HCl<br />
H 2O<br />
H +<br />
NO2<br />
NO 2<br />
NH 2<br />
Fe<br />
HCl<br />
I<br />
Fe<br />
HCl<br />
NHCOCH3<br />
(i) NaNO 2, HCl<br />
(ii)<br />
H3PO2 C2H5OH NaNO 2<br />
HCl<br />
NH 2<br />
HNO 2<br />
HCl<br />
(CH 3CO) 2O<br />
NH 2 NHCOCH3<br />
N +<br />
NCl -<br />
CH3COCl<br />
I<br />
(1) I 2<br />
H3PO2 C2H5OH NH 2 NHCOCH3<br />
CN<br />
NHCOCH 3<br />
N 2 +<br />
CuCN<br />
(2) OH - , H 2O<br />
heat<br />
H 2SO 4<br />
HNO 3
(f)<br />
NHCOCH 3<br />
NHCOCH 3<br />
CN<br />
NO2<br />
H 2SO 4<br />
HNO 3<br />
Fe<br />
HCl<br />
H 2O<br />
H +<br />
NO 2<br />
Fe<br />
HCl<br />
NHCOCH3<br />
(i) NaNO 2, HCl<br />
(ii) H3PO2 C2H5OH NH 2<br />
HNO 2<br />
HCl<br />
CH3COCl<br />
NH 2 NHCOCH3<br />
CN<br />
NHCOCH 3<br />
H 3O +<br />
N 2 +<br />
CuCN<br />
H 2SO 4<br />
HNO 3<br />
COOH<br />
20.14 Starting with p-nitroaniline [Problem 20.11(e)] show how you might synthesize<br />
1,2,3-tribromobenzene.<br />
Answer:
Br<br />
NH 2<br />
NO2<br />
CuBr<br />
Br<br />
N 2 +<br />
(1) Br 2<br />
Br<br />
Br<br />
Br<br />
Br<br />
NO 2<br />
H3PO2 C2H5OH Br<br />
NH 2<br />
NO 2<br />
Br<br />
Fe<br />
HCl<br />
Br<br />
Br<br />
NaNO 2<br />
HCl<br />
Br<br />
NH2<br />
Br<br />
Br<br />
N 2 +<br />
NO 2<br />
NaNO 2<br />
20.15 <strong>Outline</strong> a synthesis of orange Ⅱ from 2-naphthol and p-aminobenzenesulfonic acid.<br />
OH<br />
Answer:<br />
N N<br />
OrangeⅡ<br />
SO 3 - Na +<br />
Br<br />
Br<br />
HCl<br />
Br
Step1<br />
H SO HNO<br />
2N 3H 2 +N2 SO3H Step2<br />
OH<br />
+<br />
+N2 SO3H NaOH<br />
H2O OH<br />
N N<br />
SO3 - Na +<br />
20.16 Butter yellow is a dye once used to color margarine. It has since been shown to be<br />
carcinogenic, and its use in food is no longer permitted. <strong>Outline</strong> a synthesis of butter yellow from<br />
benzene and N, N-dimethylaniline.<br />
N(CH3) 2<br />
Answer:<br />
N(CH 3) 2<br />
CH 3COO - Na +<br />
N N<br />
Butter yellow<br />
HNO3<br />
H 2SO 4<br />
NO 2<br />
N N<br />
Fe<br />
H 3O +<br />
N(CH3)2<br />
NH2 HNO2<br />
20.17 Azo compounds can be reduced to amines by a variety of reagents including stannous<br />
chloride (SnCl2).<br />
SnCl2 Ar N N Ar' ArNH2 + Ar'NH2 This reduction can be useful in synthesis as the following example shows:<br />
4-Ethoxyaniline<br />
SnCl 2<br />
(1) HONO,H 3O +<br />
(2)phenol,OH -<br />
NN +<br />
A(C 14H 14N 2O 2) NaOH,CH 3CH 2Br B(C16H 18N 2O 2)<br />
two molar equivalents of C (C8H11NO) acetic anhydride phenacetin(C10H13NO2) Give a structure <strong>for</strong> phenacetin and <strong>for</strong> the intermediates A,B,C. (Phenacetin, <strong>for</strong>merly used as an
analgesic)<br />
Answer: A:<br />
H3CH2CO N N OH<br />
B:<br />
C:<br />
H3CH2CO N N OCH2CH3<br />
H 3CH 2CO NH 2<br />
Phenacetin:<br />
H 3CH 2CO NHCOCH 3<br />
20.18 An amine A has the molecular <strong>for</strong>mula C7H9N. Compound A reacts with benzene-sulfonyl<br />
chloride in aqueous potassium hydroxide to give a clear solution; acidification of the soution gives<br />
a precipitate. When A is treated with NaNO2 and HCl at 0-5℃, and then with 2-naphthol, an<br />
intensely colored compound is <strong>for</strong>med. Compound A gives a single strong IR absorption peak a<br />
815cm -1 . What is the structure of A?<br />
Answer:<br />
H3C NH2<br />
20.19 Sulfonamides of primary amines are often used to synthesize pure secondary amines.<br />
Suggest how this synthesis is carried out.<br />
Answer:<br />
O<br />
O<br />
R N S<br />
H<br />
O<br />
R N R ,<br />
H<br />
Ar<br />
O - H<br />
R , Cl<br />
O<br />
+ - O S<br />
O<br />
R N<br />
Ar<br />
R ,<br />
S<br />
O<br />
Ar<br />
+ (1)H3 O,heat<br />
(2)O-H
<strong>20.2</strong>0 (a) Starting with aniline and assuming that you have 2-aminothiazole available, show how<br />
you would synthesize sulfathiazole. (b) How would you convert sulfathiazole to<br />
succinylsulfathiazole?<br />
Answer:<br />
(a)<br />
(b)<br />
H 2N<br />
NH2<br />
NH2<br />
S<br />
SO2NH<br />
N<br />
(CH 3CO) 2O<br />
S<br />
N<br />
H 2N<br />
O<br />
NHCCH 3<br />
SO 2NH<br />
S<br />
N<br />
2-Aminothiazole<br />
O<br />
O<br />
O<br />
-HCl<br />
O<br />
NHCCH 3<br />
S<br />
N<br />
HOSO2Cl 80℃<br />
(1)dilute HCl,heat<br />
(2)HCO 3 -<br />
O<br />
O<br />
NHCCH 3<br />
SO 2Cl<br />
NH2<br />
SO2NH<br />
S<br />
Sulfathiazole<br />
NHCCH2COOH<br />
SO2NH<br />
S<br />
N<br />
Succinylsulfathiazole<br />
N
<strong>20.2</strong>1 Write structural <strong>for</strong>mulas of the following compounds:<br />
(a) Benzylmethylamine<br />
NH<br />
N<br />
N<br />
(b) Triisopropylamine<br />
(c) N-Ethyl-N-methylaniline<br />
N<br />
(d) m-Toluidine<br />
(e) 2-Methylpyrrole<br />
(f) N-Ethylpiperidine<br />
(g) N-Ethylpyridinium<br />
N<br />
H<br />
NH2
HO<br />
O<br />
O<br />
N +<br />
(i) Indole<br />
(h) 3-Pyridine carboxylic acid<br />
N<br />
(j) Acetanilide<br />
N<br />
H<br />
(k) Dimethylaminium chloride<br />
(l) 2-Mehtylimidazole<br />
(m) 3-Amino-1-propanol<br />
HO NH2 (n) Tetrapropylammonium chloride<br />
N<br />
H<br />
NH 2 + Cl -<br />
N<br />
N<br />
H
O<br />
HO<br />
N<br />
O<br />
Cl -<br />
N +<br />
(o) Pyrrolidine<br />
(p) N,N-Dimethyl-p-toluidine<br />
(q) 4-Methoxyaniline<br />
NH2<br />
(r) Tetramethylammonium hydroxide<br />
(s) p-Aminobenzoic acid<br />
(t) N-Methylaniline<br />
NH2<br />
NH<br />
OH -<br />
<strong>20.2</strong>2 Give common or systematic names <strong>for</strong> each of the following compounds:<br />
HN<br />
N +
(g)<br />
(a) CH3CH2CH2NH2 Propylamine<br />
(b) C6H5NHCH3 Methylphenylamine<br />
(c) (CH 3) 2CHN(CH 3) 3I - Isopropyl trimethyl ammonium iodide<br />
(d) o-CH3C6H4NH2 o-toluidine<br />
(e) o-CH3OC6H4NH2 o-methoxy aniline<br />
(f)<br />
N<br />
H<br />
N<br />
N NH 2<br />
N<br />
1H-Pyrazole<br />
2-amino pyrimidine<br />
(h) C 6H 5CH 2NH 3 + Cl - Benzyl-ammonium chloride<br />
(i) C6H5N(CH2CH2CH3) 2 N,N-dimethylaniline<br />
(j) C6H5SO2NH2 Benzenesulfonamide<br />
(k) CH 3NH 3 + CH3CO 2 - Methyl ammonium acetate<br />
(l) HOCH 2CH 2CH 2NH 2 3-Amino-1-propanol<br />
(m)<br />
(n)<br />
N<br />
N<br />
N<br />
H N Purine<br />
N<br />
CH 3 1-Methyl pyrrole<br />
<strong>20.2</strong>3 Show how you might prepare benzylamine from each of the following compounds:<br />
(a) Benzonitrile<br />
(b) Benzamide<br />
(c) Benzyl bromide<br />
(d) Benzyl tosylate<br />
(e) Benzaldehyde<br />
(f) Phenylnitromethane
(g) Phenlacetamide<br />
(a)<br />
(b)<br />
(c)<br />
(d)<br />
(e)<br />
(f)<br />
(g)<br />
O<br />
O<br />
C<br />
N<br />
NH 2<br />
H 2<br />
C Br<br />
O<br />
O S<br />
NH 3<br />
O<br />
NaBH 3CN<br />
N +<br />
O<br />
O -<br />
O<br />
LiAlH 4 Et 2O<br />
H 2O<br />
NH 3<br />
LiAlH 4 Et 2O<br />
H 2O<br />
Fe HCl<br />
NH 2<br />
+ Br 2 + NaOH<br />
NH 3<br />
H2<br />
C NH 2<br />
H 2<br />
C NH 2<br />
NH 2<br />
NH 2<br />
NH 2<br />
H 2<br />
C NH 2<br />
NH2<br />
+ CO 3 2-<br />
<strong>20.2</strong>4 Show how you might prepare aniline from each of the following compounds:<br />
(a) Benzene<br />
(b) Bromobenzene<br />
(c) Benzamide<br />
Answer:
(a)<br />
(b)<br />
(c)<br />
O<br />
HNO 3<br />
Br NaNH2<br />
C NH 2<br />
NH 3<br />
+ Br 2 + NaOH<br />
NO 2<br />
NH 2<br />
Fe HCl<br />
NH2<br />
NH 2<br />
+ CO 3 2-<br />
<strong>20.2</strong>5 Show how you might synthesize each of the following compounds from butyl alcohol:<br />
(a) Butylamine (free of 2 。 and 3 。<br />
(b) Pentylamine<br />
(c) Propylamine<br />
OH<br />
OH<br />
HBr<br />
amines)<br />
HBr<br />
OH Br<br />
K 2Cr 2O 7<br />
(d) Butylmethylamine<br />
NH2<br />
CH 3I<br />
O<br />
OH<br />
NH 3<br />
Br<br />
1.<br />
O<br />
O<br />
NK<br />
2. NH 2NH 2<br />
1. NaCN<br />
2. LiAlH 4<br />
O<br />
NH 2<br />
Br 2 / OH -<br />
<strong>20.2</strong>6 Show how you might convert aniline into each of the following compounds. (You need not<br />
repeat steps carried out in earlier parts of this problem.)<br />
(a) Acetanilide<br />
NH 2 NH C<br />
(CH 3CO) 2O<br />
(b) N-Phenylphthalimide<br />
O<br />
NH<br />
NH 2<br />
NH2<br />
NH 2
O<br />
O<br />
O<br />
(c) P-Nitroaniline<br />
NH C<br />
O<br />
(d) Sulfanilamide<br />
O<br />
HN<br />
C<br />
NH C<br />
C 6H 5NH 2<br />
(e) N, N-Dimethylaniline<br />
NH 2<br />
(f) Fluorobenzene<br />
HO<br />
NH2 (g) Chlorobenzene<br />
HO<br />
NH2 (h) Bromobenzene<br />
HO<br />
NH2 (i) Iodobenzene<br />
HO<br />
NH2 (j) Benzonitrile<br />
H 2SO 4/HNO 3<br />
O<br />
O<br />
S<br />
O<br />
NH 2<br />
2 CH 3I N<br />
N O<br />
0 - 5℃<br />
N O<br />
0 - 5℃<br />
N O<br />
0 - 5℃<br />
N O<br />
0 - 5℃<br />
- O<br />
O<br />
N +<br />
conc H 2SO 4<br />
HO 3S<br />
dilute HCl<br />
N 2<br />
N 2<br />
N 2<br />
N 2<br />
N<br />
O<br />
O<br />
H 2N<br />
NH<br />
HBF 4<br />
heat<br />
CuCl<br />
CuBr<br />
CuI<br />
C<br />
O<br />
NH C<br />
O<br />
NH 3<br />
H 2O<br />
O<br />
S<br />
O<br />
Cl<br />
Br<br />
I<br />
- O<br />
O<br />
NH 2<br />
F<br />
N +<br />
NH 2
HO<br />
NH2 (k) Benzoic acid<br />
(l) Phenol<br />
(m) Benzene<br />
CN<br />
HO<br />
NH2 HO<br />
NH2 N O<br />
0 - 5℃<br />
H 2O<br />
N O<br />
0 - 5℃<br />
N O<br />
0 - 5℃<br />
(n) P- (Phenylazo) phenol<br />
NH 2 NaNO 2<br />
HCl<br />
HO<br />
(o) N, N-Dimethyl-p-(phenylazo)aniline<br />
NH 2 NaNO 2<br />
HCl<br />
O<br />
N 2<br />
CuCN<br />
N 2Cu2O, Cu 2+ ,H 2O<br />
N 2<br />
N N<br />
N N<br />
H 3PO 2, H 2O<br />
OH<br />
N(CH 3) 2<br />
CN<br />
OH<br />
N<br />
N<br />
N<br />
N<br />
OH<br />
N(CH 3) 2<br />
<strong>20.2</strong>7 What products would you expect to be <strong>for</strong>med when each of the following amines reacts<br />
with aqueous sodium nitrite and hydrochloric acid?<br />
(a) Propylamine (c)N-Propylaniline (e)p-Propylaniline<br />
(b)Dipropylamine (d)N,N-Dipropylaniline<br />
Answer:<br />
(a)
CH 3CH 2CH 2 NH 2<br />
CH 3CH 2CH 2OH<br />
(b)<br />
NaNO 2<br />
HCl<br />
CH 3CH 2CH 2<br />
CH 3CH 2CH 2 N N<br />
CH3CH=CH2 CH3CH2CH2Cl CH 3CH 2CH 2 NH NaNO 2<br />
HCl<br />
H3CH2CH2C H3CH2CH2C<br />
(c)<br />
(d)<br />
HN<br />
H 3CH 2CH 2C<br />
(e)<br />
NH 2<br />
CH 2CH 2CH 3<br />
N<br />
CH2CH2CH3<br />
NaNO2<br />
HCl<br />
CH 2CH 2CH 3<br />
NaNO2<br />
HCl<br />
O<br />
NaNO2<br />
HCl<br />
N<br />
CH 3CH 2CH 2<br />
N<br />
H3CH2CH2C<br />
N<br />
N<br />
CH 3CHCH 3<br />
CH 3CHClCH 3<br />
CH 2CH 2CH 3<br />
N<br />
N O<br />
CH 2CH 2CH 3<br />
N N<br />
CH2CH2CH3<br />
CH 3CH 2OHCH 3<br />
<strong>20.2</strong>8 (a) What products would you expect to be <strong>for</strong>med when each of the following<br />
O
Answer:<br />
NH2<br />
amines in the preceding problem reacts with benzenesulfonyl chloride and excess<br />
aqueous potassium hydroxide? (b) What would you observe in each reaction? (c)<br />
What would you observe when the resulting solution or <strong>mixture</strong> is acidified?<br />
CH2CH2CH3<br />
H3CH2CH2C<br />
HN<br />
N<br />
TsCl<br />
KOH<br />
CH 2CH 2CH 3<br />
CH2CH2CH3<br />
TsCl<br />
KOH<br />
Ts<br />
TsCl<br />
KOH<br />
N<br />
CH 2CH 2CH 3<br />
CH3CH2CH2 NH<br />
KOH<br />
H3CH2CH2C H3CH2CH2C<br />
CH 3CH 2CH 2 NH 2<br />
TsCl<br />
KOH<br />
TsCl<br />
Ts<br />
N<br />
HA<br />
No reaction<br />
CH 2CH 2CH 3<br />
CH 3CH 2CH 2<br />
CH 3CH 2CH 2 N<br />
Ts<br />
N<br />
HA<br />
Ts<br />
Ts<br />
NH<br />
CH 2CH 2CH 3<br />
CH 3CH 2CH 2 NH<br />
<strong>20.2</strong>9 (a) What product would you expect to obtain from the reaction of piperidine with aqueous<br />
sodium nitrite and hydrochloric acid? (b) From the reaction of piperidine and benzenesulfonyl<br />
chloride in excess aqueous potassium hydroxide?<br />
Answer:<br />
Ts
(a)<br />
N<br />
N<br />
O (b)<br />
20.30 Give structure <strong>for</strong> the products of each of the following reactions:<br />
(a) Ethylamine + benzoyl chloride O<br />
(b) Methylamine + acetic anhydride<br />
(c) Methylamine + succinic anhydride O<br />
(d) Product of (c)<br />
O<br />
(e) Pyrrolidine + phthalic anhydride O O<br />
(f) Pyrrole +acetic anhydride<br />
N<br />
O<br />
S<br />
O<br />
HN<br />
N<br />
N<br />
H<br />
O<br />
N<br />
O<br />
N<br />
O<br />
O<br />
HN<br />
OH<br />
OH
(g) Aniline + propanoyl chloride<br />
(h) Tetraethylammonium hydroxide<br />
(i)<br />
m-Dinitrobenzene + H 2S<br />
p-Toluidine + Br 2(excess) H2O<br />
NH 3<br />
C 2H 5OH<br />
(j)<br />
Br<br />
20.31 Starting with benzene or toulene, outline a synthesis of each of the following compounds<br />
using diazonium salts as intermediates. (You need not repeat syntheses carried out in earlier of this<br />
proplem.)<br />
(a) p-Fluorotoluene<br />
(b) o-Iodotoluene<br />
(c) p-Cresol<br />
(d) m-Diachlorobenzene<br />
(e) m-(C6H4(CN)2<br />
(f) m-Iodophenol<br />
(g) m-Bromobenzenontrile<br />
(h) 1,3-Dibromo-5-nitrobenzene<br />
(i) 3,5-Dibromoaniline<br />
(j) 3,4,5-TriBromophenol<br />
(k) 3,4,5-Tribromobenzonitrile<br />
(l) 2,6-Dibromobenzoic acid<br />
(m) 1,3-Dibromo-2-iodobenzene<br />
(n) 4-Bromo-2-nitrotoluene<br />
(o) 4-Methyl-3-nitrophenol<br />
O<br />
O<br />
N<br />
Br<br />
O<br />
HN<br />
N<br />
NH 2<br />
NH 2
H 3C<br />
(q)<br />
H3C<br />
(r)<br />
H3C<br />
NC<br />
Answer:<br />
(a)<br />
(b)<br />
(c)<br />
H 2N<br />
(d)<br />
CH3<br />
heat<br />
HNO 3<br />
H 2SO 4/warm<br />
F<br />
HNO<br />
CH3<br />
3<br />
H2SO4/warm CH 3<br />
Br<br />
N N<br />
N N<br />
O 2N<br />
NO 2<br />
CH 3<br />
CH 3<br />
HONO/H 2SO 4<br />
OH<br />
CH 3<br />
CH 3<br />
Fe/HCl<br />
OH<br />
H 2N<br />
Fe/HCl<br />
CH3<br />
NH2 HONO<br />
HCl<br />
O 4SHN 2<br />
CH 3<br />
CH 3<br />
Cu,Cu 2O,H 2O<br />
(1)HONO,H<br />
(2)HBF4 HO<br />
N2Cl<br />
CH 3<br />
F 4BN 2<br />
CH 3<br />
KI CH 3<br />
CH 3<br />
I
(e)<br />
(f)<br />
O2N<br />
(g)<br />
O 2N<br />
(h)<br />
O 2N<br />
(i)<br />
Br<br />
O 2N<br />
(j)<br />
(k)<br />
HNO 3<br />
H 2SO 4<br />
HONO,HCl<br />
HNO3 NO2 NO2<br />
H2SO4 NH 2<br />
NH 2<br />
NH 2<br />
H3O heat<br />
NO 2<br />
Br<br />
O 2N<br />
HONO/HI<br />
O2N<br />
NO 2<br />
Cl<br />
Cl 2/FeCl 3<br />
Fe/HCl<br />
N 2I<br />
KI<br />
H 2N<br />
O2N<br />
Cl<br />
N 2Cl<br />
NH 2<br />
CuCl<br />
I<br />
NO 2<br />
Cl<br />
HCl,HONO 2<br />
ClN 2<br />
Fe/HCl<br />
Cl<br />
Cl<br />
N 2Cl<br />
CuCN<br />
NH 2<br />
NC<br />
Fe/HCl (1)HCl,HONO2 I<br />
(2)Cu,Cu2O,H2O HONO/HBr CuBr<br />
Zn/HCl (1)HCl,HONO2 N2Br Br<br />
Br<br />
(2)CuCN<br />
Br 2/FeBr 3<br />
CH 3COCl<br />
O 2N<br />
Fe/HCl<br />
Br<br />
O 2N<br />
Br<br />
Br<br />
NH 2<br />
O 2N<br />
H2S,NH3,C2H5OH NO2<br />
Br<br />
O2N NH2 (1)HBr,HONO<br />
(2)CuBr<br />
Br<br />
Br<br />
O 2N<br />
H 2N<br />
H 2N<br />
O<br />
HNO3 NHCCH3 H2SO4/warm O2N<br />
O<br />
NHCCH3<br />
NH2<br />
(1)HBr,HONO<br />
(2)CuBr<br />
Br<br />
O 2N<br />
HO<br />
NC<br />
CN<br />
I<br />
Br<br />
Br<br />
Br 2/FeBr 3 O2N NHCCH 3<br />
Br<br />
Br<br />
(1)Zn/HCl<br />
(2)HI,HONO<br />
O2N Br<br />
HO Br<br />
(3) Cu2O, Cu2+, H2O Br<br />
Br<br />
Br<br />
Br<br />
O
Br<br />
O 2N Br<br />
(l)<br />
(m)<br />
Br<br />
(1)Zn/HCl<br />
(2)HCl,HONO<br />
(3)CuCN<br />
Br<br />
NC Br<br />
HNO3 Br2/FeBr3 CH3 O2N CH3 O2N<br />
CH3 H2SO4/warm (1)HCl,HONO<br />
(2)H3PO2/heat O 2N NH 2<br />
(n)<br />
Br<br />
Br<br />
(1)HCl,HONO<br />
(2)H3PO2,H2O H 2N<br />
(o)<br />
O2N<br />
(p)<br />
Br<br />
(q)<br />
CH 3<br />
Br<br />
Br<br />
(1)HI,HONO<br />
(2)KBr<br />
Br<br />
Br<br />
I<br />
CH 3<br />
(1)HBr,HONO<br />
(2)CuBr<br />
KMnO 4<br />
Br<br />
Br<br />
Br<br />
Br<br />
O 2N I<br />
NO 2 NO 2<br />
HNO3/H2SO4 CH3<br />
O2N NO2<br />
CH3<br />
NO 2<br />
CH 3<br />
Br<br />
H 2S, NH 3<br />
H2N<br />
Br<br />
Br<br />
Br<br />
COOH<br />
Zn/HCl<br />
NO 2<br />
CH 3<br />
Zn/HCl<br />
H2N<br />
Br<br />
Br<br />
Br<br />
CH 3<br />
H 2N I<br />
(1)HCl,HONO<br />
CH3<br />
HO<br />
(2)Cu,Cu2O,H2O Zn/HCl (1)HCl,HONO<br />
Br<br />
CH3 Br<br />
(2)CuCN<br />
NH 2<br />
Br<br />
CN<br />
NO2<br />
CH3<br />
CH 3
(r)<br />
(1)H2SO4/HONO NH2<br />
(2)Cu,CU2O,H2O OH<br />
ClN 2<br />
ClN2 CH3 + H3C<br />
OH<br />
20.32 Write equations <strong>for</strong> simple chemical tests that would distinguish between<br />
(a) Benzylamine and benzamide<br />
(b) Allyamine and popylamine<br />
(c) P-Toluidine and N-methylaniline<br />
(d) Cyclohexylamine and piperidine<br />
(e) Pyridine and benzene<br />
(f) Cyclohexylamine and aniline<br />
(g) Triethylamine and diethylamine<br />
(h) Tripropylammonium chloride and tetrapropylammonium chloride<br />
(i) Tetrapropylammonium chloride and tetrapropylammonium hydroxide<br />
Answer:<br />
(a)<br />
(b)<br />
NH 2<br />
CONH 2<br />
Br 2<br />
Br 2<br />
Br<br />
NH 2<br />
Br<br />
no reaction<br />
Br<br />
CH 3<br />
H3C<br />
H 3C<br />
N N<br />
N N<br />
OH<br />
CH 3<br />
OH
(c)<br />
(d)<br />
(e)<br />
(f)<br />
NH 2<br />
CH 3<br />
NHCH 3<br />
NH2<br />
H<br />
N<br />
N<br />
NH 2<br />
NH 2<br />
PhSO 2Cl<br />
PhSO 2Cl<br />
HCl/H 2O<br />
Br 2<br />
Br 2<br />
Br<br />
NHSO 2Ph<br />
CH 3<br />
H 3CN SO 2Ph<br />
NHSO 2Ph<br />
SO 2Ph<br />
N<br />
N<br />
H<br />
Br<br />
no reaction<br />
Cl<br />
no reaction<br />
NaOH<br />
NaOH<br />
NH 2<br />
NSO2Ph<br />
CH3<br />
no reaction<br />
NSO 2Ph<br />
no reaction
(g)<br />
(h)<br />
NH2<br />
NH 2<br />
N<br />
NH<br />
Br 2<br />
NHCl<br />
NCl<br />
PhSO 2Cl<br />
no reaction<br />
NH 2<br />
Br Br<br />
NaOH<br />
Br<br />
no reaction<br />
NSO 2Ph<br />
no reaction<br />
(i)<br />
Tetrapropylammonium chloride dissolves in water to give a neutral solution, however,<br />
tetrapropylammonium hydroxide gives a strong basic solution.<br />
20.33 Describe with equations how you might separate a <strong>mixture</strong> of aniline, p-cresol, benzoic acid,<br />
and toluene using ordinary laboratory reagents.<br />
Solution:<br />
N
<strong>mixture</strong> H+<br />
separation<br />
NH3<br />
OH -<br />
<strong>mixture</strong> OH- separation<br />
NH2<br />
H +<br />
CH 3<br />
NaHCO 3<br />
separation<br />
OH<br />
CH 3<br />
COONa<br />
20.34 Show how you might synthesize β-aminopropionic acid (H 3NCH 2CH 2CO 2 - ) from<br />
succinic anhydride. (β-Aminopropionic acid is used in the synthesis of pantothenic acid; see<br />
Problem 18.34.)<br />
Solution:<br />
O<br />
O<br />
O<br />
O<br />
+ NH 3<br />
H 2C<br />
C<br />
H 2C C<br />
O<br />
NH 2 Br/OH -<br />
OH<br />
H 2C NH 2<br />
H 2C<br />
C<br />
O<br />
O<br />
H +<br />
H +<br />
COOH<br />
H 3NCH 2CH 2CO 2<br />
20.35 Show how you might synthesize each of the following <strong>for</strong>m the compounds indicated and<br />
any other needed reagents.<br />
(a) (CH3)3 + N(CH2)10 + N(CH3)32Br - from 1,10-decanediol<br />
(b) Succinylcholine bromide (see the chapter opening vignette) from succinic acid,<br />
2-bromoethanol, and trimethylamine<br />
Answers:<br />
(a)<br />
(b)<br />
HO(CH 2) 10OH 2HBr Br(CH 2) 10Br 2N(CH 3) 3 H3C N<br />
HOOC<br />
2N(CH 3) 3<br />
COOH<br />
2BrCH 2CH 2OH<br />
O O<br />
CH 3<br />
CH 3<br />
(CH3) 3N(CH2) 2OC(CH2) 2CO(CH2) 2N(CH3) 3 2Br<br />
(CH 2) 10N<br />
CH 3<br />
CH 3<br />
O O<br />
2Br<br />
CH 3<br />
Br(CH 2) 2OC(CH 2) 2CO(CH 2) 2Br
20.36 A commercial synthesis of folic acid consists of heating the following three compounds with<br />
aqueous sodium bicarbonate. Propose reasonable mechanisms <strong>for</strong> the reactions that lead to folic<br />
acid.<br />
Answers:<br />
H 2N<br />
H 2N<br />
N<br />
N<br />
N<br />
OH<br />
H2N<br />
N<br />
OH<br />
N<br />
NH 2<br />
NH 2<br />
N<br />
N<br />
+<br />
N<br />
OH<br />
Br<br />
O<br />
NH2<br />
NH2<br />
Br<br />
Br<br />
+<br />
O<br />
CHBr 2CCH 2Br<br />
H 2N<br />
COOH<br />
+ H 2N CONHCHCH 2CH 2COOH<br />
HCO 3,H 2O<br />
Folic acid (~10%)<br />
N<br />
N<br />
OH<br />
NH 2<br />
H<br />
N CONHCHCH 2CH 2COOH<br />
COOH<br />
20.37 When compound W(C15H17N) is treated with benzenesulfonyl chloride and aqueous<br />
potassium hydroxide, no apparent change occurs. Acidification of this <strong>mixture</strong> gives a clear<br />
solution. The 1 H NMR spectrum of W is shown in Fig.20.7. Propose a structure <strong>for</strong> W.<br />
Answer:<br />
N<br />
N-benzyl-N-ethylaniline<br />
20.38 Propose structures <strong>for</strong> compounds X, Y, and Z.<br />
X(C 7H 7Br) NaCN<br />
Y(C 8H 7N) LiAlH 4<br />
Z(C 8H 11N)<br />
The 1 H NMR spectrum of X gives two signals, a multiplet at δ7.3(5H) and a singlet at δ<br />
4.25(2H); the 680-840-cm -1 region of the IR spectrum of Y is similar to that of X: multiplet at δ<br />
7.3(5H), singlet at δ3.7(2H). The 1 H NMR spectrum of Z is shown in Fig.20.8.<br />
Answer:<br />
X<br />
Br<br />
; Y<br />
CN<br />
; Z<br />
N<br />
Br<br />
Br<br />
Br<br />
NH 2<br />
H 2N<br />
.<br />
H 2N<br />
N<br />
N<br />
N<br />
OH<br />
N<br />
OH<br />
H<br />
N<br />
N<br />
N<br />
N<br />
HCO 3 -<br />
Br<br />
Br<br />
Br
20.39 Using reactions that we have studied in this chapter, propose a mechanism that accounts <strong>for</strong><br />
the following reaction:<br />
O<br />
Answer:<br />
O<br />
CH2CH2CN<br />
CH2CH2CN<br />
H 2,Pd<br />
H 2,Pd<br />
20.40 Give structures <strong>for</strong> compounds R-W:<br />
O<br />
H<br />
N<br />
-H 2 O<br />
N - Methylpiperidine + CH 3I R(C 7H 16NI) Ag 2O<br />
H 2O<br />
T(C 7H 15N)<br />
Answer:<br />
R:<br />
U:<br />
N I<br />
N I<br />
CH 3I<br />
U(C 8H 19NO)<br />
S:<br />
V:<br />
H<br />
Ag 2O<br />
H 2O<br />
N OH<br />
N OH<br />
V(C 8H 19NO)<br />
T:<br />
N<br />
H<br />
N<br />
H 2,Pd<br />
S(C 7H 17NO)<br />
heat W(C 5H 8+H 2O+(CH 3) 3N)<br />
20.41 Compound A (C10H15N) is soluble in dilute HCl. The IR absorption spectrum shows two<br />
bands in the 3300-3500cm -1 region. The broadband proton-decoupled 13 C spectrum of A is given<br />
in Fig. 20.9. Propose a structure <strong>for</strong> A.<br />
Answer:<br />
W:<br />
N<br />
H<br />
N
H 3CH 2C<br />
NH 2<br />
CH 2CH 3<br />
20.42 Compound B, an isomer of (Problem 20.41), is also soluble in dilute HCl. The IR<br />
spectrum of B shows no bands in the 3300-3500 cm -1 region. The broadband proton-decoupled<br />
13<br />
C spectrum of B is given in Fig. 20.9. Propose a structure <strong>for</strong> B.<br />
Answer:<br />
H 3CH 2C<br />
N<br />
CH 2CH 3<br />
20.43 Compound C (C9H11NO) gives a positive Tollens’ test and is soluble in dilute HCl. The IR<br />
spectrum of C shows a strong band near 1695 cm -1 but shows no bands in the 3300-3500 cm -1<br />
region. The broadband proton-decoupled 13 C NMR spectrum of C is shown in Fig. 20.9. Propose a<br />
structure <strong>for</strong> C.<br />
Answer:<br />
CHO<br />
N<br />
H3C CH3 20.44 <strong>Outline</strong> a synthesis of acetylcholine iodide using as organic starting materials:<br />
dimethylamine, oxirane, and acetyl chloride.
H 3C N<br />
Answer:<br />
H 3C<br />
H<br />
N<br />
CH3<br />
CH3<br />
H 2<br />
C<br />
I<br />
H 2<br />
C O<br />
Acetylcholine iodide<br />
CH 3<br />
H 2C<br />
O<br />
O<br />
C<br />
CH2 H 3C N<br />
CH 3I<br />
CH3<br />
CH3<br />
H 3C N<br />
CH3<br />
CH3<br />
CH3COCl CH2CH2OH H3C N<br />
CH2CH2O<br />
I<br />
O<br />
C<br />
CH3<br />
CH3<br />
CH2CH2O<br />
20.45 Ethanolamine, HOCH2CH2NH2, and diethanolamine, (OHCH2CH2)2NH, are used<br />
commercially to <strong>for</strong>m emulsifying agents and to absorb acidic gases. Propose syntheses of these<br />
two compounds.<br />
Answer:<br />
O<br />
+ NH HOCH 3 2CH2NH2 O<br />
+ HOCH2CH2NH2 (HOCH2CH2) 2NH<br />
20.46 Diethylpropion (see the following structure) is a compound used in the treatment of<br />
anorexia. Propose a synthesis of diethylpropion starting with benzene and using any other needed<br />
reagents.<br />
Answer:<br />
O<br />
C<br />
CH3
NH(C 2H 5) 2<br />
CH 3CH 2COCl<br />
AlCl 3<br />
O<br />
O<br />
N(C2H5)2<br />
CH 2CH 3<br />
Br 2<br />
O<br />
CHCH 3<br />
20.47 Suggest an experiment to test the proposition that the Hofmann reaction is an intramolecular<br />
rearrangenment, i.e.,one in which the migrating R group never fully separates from the amide<br />
molecule.<br />
A:<br />
C 6H 5H 2C<br />
O<br />
CNH2<br />
Br 2, NaOH, H 2O<br />
C6H5H2C<br />
H3C H<br />
H3C<br />
H<br />
From the example above, the R group migrates to nitrogen with its electrons, but without<br />
inversion.<br />
20.48 Using as starting materials propanoic acid, aniline, and 2-naphthol, propose a synthesis of<br />
naproanilide, a herbicide used in rice paddies in the Orient.<br />
OH<br />
O<br />
OH<br />
Cl 2<br />
P<br />
Cl<br />
Cl<br />
O<br />
O<br />
Cl<br />
NH 2<br />
20.49 When phenyl is isothiocyanate, C6H5N=C=S, is reduced with lithium aluminum hydride,<br />
the product <strong>for</strong>med has these spectral data:<br />
MS (m/z): 107, 106<br />
IR (cm -1 ): 3330(sharp), 3050, 2815, 760, 700<br />
1<br />
H NMR (δ ): 2.7(s), 3.5(board), 6.6(m), 7.2(t)<br />
13<br />
C NMR (δ ): 30(CH2), 112(CH), 117(CH), 129(CH2), 150(C)<br />
(a) What is the structure of the product?<br />
(b) What is the structure that account <strong>for</strong> the 106m/z peak and how is it <strong>for</strong>med? (It is an iminium<br />
O<br />
O<br />
Cl<br />
N<br />
H<br />
H<br />
N<br />
NH 2<br />
Br
ion.)<br />
Answer:<br />
(a)<br />
(b)<br />
H<br />
N<br />
H<br />
N<br />
CH 3<br />
CH 3<br />
-e -<br />
H<br />
Ph N CH2<br />
H<br />
m / z 107<br />
- H 2O<br />
Ph N CH2<br />
H<br />
m / z 106<br />
*20.50 When N,N’-diphenylurea (A) is reacted with tosyl chloride in pyridine, it yields product B.<br />
H<br />
N<br />
O<br />
A<br />
H<br />
N<br />
The spectral data <strong>for</strong> B include:<br />
MS (m/z): 194 (M + )<br />
IR (cm -1 ): 3060, 2130, 1590, 1490, 760, 700<br />
1<br />
H NMR (δ): only 6.9-7.4 (m)<br />
13<br />
C NMR (δ): 122 (CH), 127 (CH), 130 (CH), 149 (C), and 163 (C)<br />
(a) What is the structure of B?<br />
(b) Write a mechanism <strong>for</strong> <strong>for</strong>mation of B.<br />
Answer:<br />
N C N
H<br />
N<br />
O<br />
A<br />
H<br />
N<br />
H<br />
N N<br />
OH<br />
N C N<br />
20.51 Propose a mechanism that can explain occurrence of this reaction:<br />
N<br />
O<br />
Answer:<br />
N<br />
O<br />
COOH<br />
COOH<br />
N<br />
O<br />
+<br />
H<br />
O O O<br />
N<br />
O<br />
COOH<br />
O<br />
O<br />
N<br />
O<br />
O<br />
H<br />
C<br />
COOH O O O<br />
O<br />
O<br />
+<br />
CH3<br />
N<br />
O<br />
O<br />
C<br />
O<br />
N<br />
O<br />
O O<br />
O<br />
O<br />
H<br />
TsCl<br />
N<br />
O<br />
O<br />
O<br />
CH 3<br />
H<br />
N N<br />
N<br />
OTs<br />
C<br />
COOH<br />
O<br />
O O<br />
20.52 When acetone is treated with anhydrous ammonia in the presence of anhydrous calcium<br />
chloride (a common drying agent), crystalline product C is obtained on concentration of the<br />
organic liquid phase of the reaction <strong>mixture</strong>.<br />
These are spectral data <strong>for</strong> product C:<br />
MS (m/z): 155 (M + ·), 140<br />
IR (cm -1 ): 3350 (sharp), 2850-2960, 1705<br />
1<br />
H NMR (δ): 2.3 (s, 4H), 1.7 (1H; disappears in D2O), and 1.2 (s, 12H)<br />
(a) What is the structure of C?<br />
N<br />
O<br />
O<br />
C<br />
O<br />
H<br />
N<br />
O<br />
H<br />
O
(b) Propose a mechanism <strong>for</strong> <strong>for</strong>mation of C.<br />
Answer:<br />
HN O<br />
OH<br />
NH<br />
O<br />
NH2<br />
O<br />
CH3<br />
N<br />
CH 3<br />
O<br />
N<br />
CH 2<br />
OH<br />
HN O