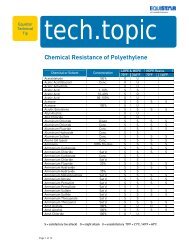
Film Extrusion Guide.pmd - LyondellBasell
Film Extrusion Guide.pmd - LyondellBasell
Film Extrusion Guide.pmd - LyondellBasell
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
H H<br />
C = C<br />
H H<br />
Figure 1. Ethylene monomer<br />
molecular structure<br />
H H H H H H H H H H<br />
C C C C C C C C C C<br />
H H H H H H H H H H<br />
Figure 2. Polyethylene molecular<br />
chain.<br />
Figure 3. Polyethylene chain with<br />
side branches.<br />
Figure 4. Polyethylene chain with<br />
side branches.<br />
Polyolefins Are<br />
Thermoplastics Derived<br />
From Petrochemicals<br />
Polyolefins are plastic resins<br />
polymerized from petroleum-based<br />
gases. The two principal gases are<br />
ethylene and propylene. Ethylene is<br />
the principal raw material for<br />
making polyethylene (PE) and<br />
ethylene copolymer resins; and<br />
propylene is the main ingredient for<br />
making polypropylene (PP) and<br />
propylene copolymer resins.<br />
Polyolefin resins are classified<br />
as thermoplastics, which means<br />
that they can be melted, solidified<br />
and melted again. This contrasts<br />
with thermoset resins which, once<br />
molded, cannot be reprocessed.<br />
Most polyolefin resins for film<br />
extrusion generally are used in<br />
pellet form. The pellets are about<br />
1/8 inch thick and 3/16 inch in<br />
diameter, usually somewhat<br />
translucent and white in color.<br />
Polyolefin resins sometimes will<br />
contain additives, such as thermal<br />
stabilizers. They also can be<br />
compounded with colorants,<br />
antistatic agents, slip and antiblock<br />
additives, UV stabilizers, etc.<br />
Molecular Structure and<br />
Composition Affect Properties<br />
and Processability<br />
Three basic molecular<br />
properties affect most of the<br />
properties essential to high quality<br />
film extrusion:<br />
• Average Molecular Weight<br />
• Molecular Weight Distribution<br />
• Crystallinity or Density.<br />
These molecular properties are<br />
determined by the materials used<br />
to produce the polyolefins and the<br />
conditions under which they are<br />
manufactured. The basic building<br />
6<br />
blocks for the gases from which<br />
polyolefins are derived are hydrogen<br />
and carbon atoms. For polyethylenes,<br />
these atoms are combined<br />
to form the ethylene monomer,<br />
C 2 H 4 , i.e., two carbon atoms and<br />
four hydrogen atoms (see Figure<br />
1). In the polymerization process,<br />
the double bond connecting the<br />
carbon atoms is broken. Under the<br />
right conditions, these bonds<br />
reform with other ethylene molecules<br />
to form long molecular<br />
chains (Figure 2). The resulting<br />
product is polyethylene.<br />
For polypropylene, the<br />
hydrogen and carbon atoms are<br />
combined to form the propylene<br />
monomer, CH 3 CH=CH 2 , which has<br />
three carbon atoms and six<br />
hydrogen atoms (Figure 3). The<br />
third carbon atom remains pendant<br />
and protrudes from the spiraling<br />
backbone chain.<br />
Ethylene copolymers, such as<br />
EVA and EMA, are made by the<br />
polymerization of ethylene units<br />
with randomly distributed<br />
comonomer groups, such as vinyl<br />
acetate (VA) and methyl acrylate<br />
(MA).<br />
The polymerization of<br />
monomers creates a mixture of<br />
molecular chains of varying<br />
lengths. Some are short, while<br />
others are enormously long,<br />
containing several hundred<br />
thousand monomer units. For<br />
polyethylene, the ethylene chains<br />
have numerous side branches. For<br />
every 100 ethylene units in the<br />
molecular chain, there are about<br />
one to 10 short or long branches.<br />
The branches radiate in three<br />
dimensions (Figure 4).<br />
Chain branching affects many<br />
polymer properties including<br />
density, hardness, flexibility and<br />
transparency, to name a few. Chain<br />
branches also become points in the<br />
molecular network where oxidation<br />
may occur. In some processing<br />
techniques where high temperatures<br />
are reached, oxidation can<br />
adversely affect the polymer’s<br />
properties.