Drug Disposition Overview - Pharmacology and at UCSD
Drug Disposition Overview - Pharmacology and at UCSD
Drug Disposition Overview - Pharmacology and at UCSD
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
BIOM/PHAR 255 Winter 2013 Halpert - Jan. 17, 2013<br />
Human cytosolic sulfotransferases<br />
SULT Substr<strong>at</strong>es Remarks<br />
1A1<br />
1A2<br />
simple phenols, Minoxidil,<br />
acetaminophen, N-OHPhIP<br />
N-OH-AAF, simple phenols<br />
Most abundant 1A form in liver<br />
Only found in humans. Protein may not be<br />
expressed.<br />
1A3 Dopamine, other c<strong>at</strong>echolamines Low in liver, high in intestine, placenta<br />
1A4<br />
1B1<br />
C<strong>at</strong>echolamines<br />
Thyroid hormones Liver, intestine, leukocytes<br />
1C2 N-OH-AAF, simple phenols<br />
1C4 N-OH-AAF, simple phenols Highest in fetal liver, kidney<br />
Highest in fetal liver, kidney<br />
1E1 estrogens Liver, intestine, secretory endometrium<br />
Inhibited by PCB metabolites<br />
2A1 Steroids, DHEA, hydroxymethyl<br />
PAHs<br />
Liver, intestine, steroidogenic organs<br />
2B1 Steroids, cholesterol<br />
2 variants with altern<strong>at</strong>ive exons 1<br />
4A1 ?<br />
2 variants. High in brain<br />
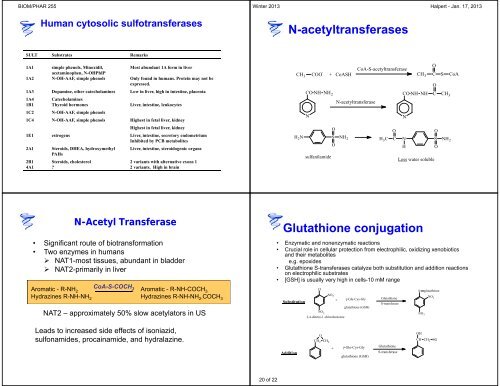
N-Acetyl Transferase<br />
• Significant route of biotransform<strong>at</strong>ion<br />
• Two enzymes in humans<br />
NAT1-most tissues, abundant in bladder<br />
NAT2-primarily in liver<br />
Arom<strong>at</strong>ic - R-NH 2<br />
Hydrazines R-NH-NH 2<br />
CoA-S-COCH 3<br />
Arom<strong>at</strong>ic - R-NH-COCH 3<br />
Hydrazines R-NH-NH 2-COCH 3<br />
NAT2 – approxim<strong>at</strong>ely 50% slow acetyl<strong>at</strong>ors in US<br />
Leads to increased side effects of isoniazid,<br />
sulfonamides, procainamide, <strong>and</strong> hydralazine.<br />
20 of 22<br />
N-acetyltransferases<br />
CH 3<br />
N<br />
COO -<br />
CO NH NH 2<br />
H 2N S NH 2<br />
sulfanilamide<br />
O<br />
CoA-S-acetyltransferase<br />
+ CoASH CH3 C<br />
O<br />
O<br />
N-acetyltransferase<br />
H 3 C<br />
O<br />
C<br />
CO NH NH C<br />
N<br />
O<br />
O<br />
S CoA<br />
CH 3<br />
N S NH 2<br />
H<br />
Less w<strong>at</strong>er soluble<br />
Glut<strong>at</strong>hione conjug<strong>at</strong>ion<br />
• Enzym<strong>at</strong>ic <strong>and</strong> nonenzym<strong>at</strong>ic reactions<br />
• Crucial role in cellular protection from electrophilic, oxidizing xenobiotics<br />
<strong>and</strong> their metabolites<br />
e.g. epoxides<br />
• Glut<strong>at</strong>hione S-transferases c<strong>at</strong>alyze both substitution <strong>and</strong> addition reactions<br />
on electrophilic substr<strong>at</strong>es<br />
• [GSH] is usually very high in cells-10 mM range<br />
Substitution<br />
Addition<br />
Cl<br />
NO 2<br />
+<br />
γ-Glu-Cys-Gly<br />
glut<strong>at</strong>hione (GSH)<br />
NO2 2,4-dinitro-1-chlorobenzene<br />
O<br />
CH2 CH2 +<br />
γ-Glu-Cys-Gly<br />
glut<strong>at</strong>hione (GSH)<br />
Glut<strong>at</strong>hione<br />
S-transferase<br />
Glut<strong>at</strong>hione<br />
S-transferase<br />
NO 2<br />
O<br />
S glut<strong>at</strong>hione<br />
NO2 OH<br />
CH CH2 SG