TNT: Trinitrotoluenes and Mono and Dinitrotoluenes
TNT: Trinitrotoluenes and Mono and Dinitrotoluenes
TNT: Trinitrotoluenes and Mono and Dinitrotoluenes
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
THE THEORETICAL NITRATION OF TOLUENE 23<br />
nitro-group can then easily replace the sulphonic<br />
group.<br />
The formation of the small amount of meta nitrotoluene<br />
is explained thus by Holleman: (2) "It is<br />
apparent that there is an opposition between the ortho<br />
<strong>and</strong> para derivatives on one h<strong>and</strong>, <strong>and</strong> the meta derivatives<br />
on the other. Either tjhe first two are the chief<br />
products, or the last. Concerning the temperature<br />
of the reaction, it has been proved that the quantity<br />
of the by-products is the smaller, the lower the temperature<br />
of nitration." This statement of Holleman<br />
is based on earlier work by himself, <strong>and</strong> in a paper he<br />
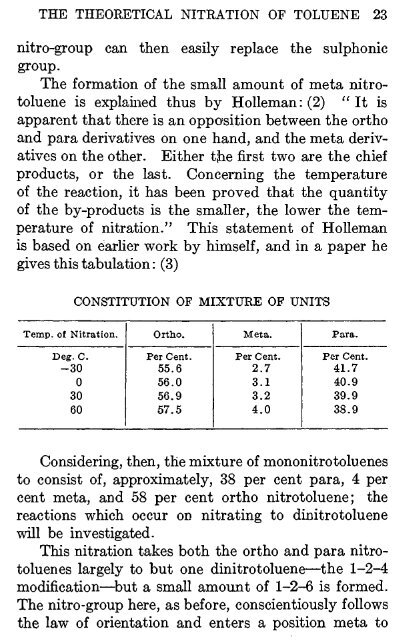
gives this tabulation: (3)<br />
Temp, of Nitration.<br />
Deg. C.<br />
-30 0<br />
30<br />
60<br />
CONSTITUTION OF MIXTURE OF UNITS<br />
Ortho.<br />
Percent.<br />
55.6<br />
56.0<br />
56.9<br />
57.5<br />
Meta.<br />
Per Cent.<br />
2.7<br />
3.1<br />
3.2<br />
4.0<br />
Para.<br />
Per Cent.<br />
41.7<br />
40.9<br />
39.9<br />
38.9<br />
Considering, then, the mixture of mononitrotoluenes<br />
to consist of, approximately, 38 per cent para, 4 per<br />
cent meta, <strong>and</strong> 58 per cent ortho nitrotoluene; the<br />
reactions which occur on nitrating to dinitrotoluene<br />
will be investigated.<br />
This nitration takes both the ortho <strong>and</strong> para nitrotoluenes<br />
largely to but one dinitrotoluene—the 1-2-4<br />
modification—but a small amount of 1-2-6 is formed.<br />
The nitro-group here, as before, conscientiously follows<br />
the law of orientation <strong>and</strong> enters a position meta to