TNT: Trinitrotoluenes and Mono and Dinitrotoluenes
TNT: Trinitrotoluenes and Mono and Dinitrotoluenes
TNT: Trinitrotoluenes and Mono and Dinitrotoluenes
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
INTRODUCTION 3<br />
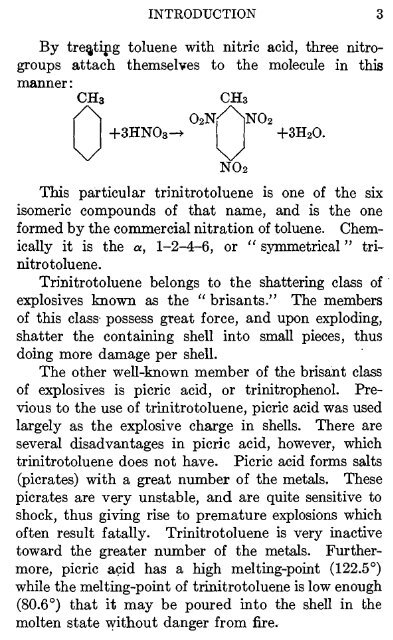
By treating toluene with nitric acid, three nitrogroups<br />
attach themselves to the molecule in this<br />
manner:<br />
CH3<br />
CH3<br />
O2N/NNO2<br />
+3HN03-> +3H2O.<br />
NO2<br />
This particular trinitrotoluene is one of the six<br />
isomeric compounds of that name, <strong>and</strong> is the one<br />
formed by the commercial nitration of toluene. Chemically<br />
it is the a, 1-2-4-6, or " symmetrical" trinitrotoluene.<br />
Trinitrotoluene belongs to the shattering class of<br />
explosives known as the " brisants." The members<br />
of this class possess great force, <strong>and</strong> upon exploding,<br />
shatter the containing shell into small pieces, thus<br />
doing more damage per shell.<br />
The other well-known member of the brisant class<br />
of explosives is picric acid, or trinitrophenol. Previous<br />
to the use of trinitrotoluene, picric acid was used<br />
largely as the explosive charge in shells. There are<br />
several disadvantages in picric acid, however, which<br />
trinitrotoluene does not have. Picric acid forms salts<br />
(picrates) with a great number of the metals. These<br />
picrates are very unstable, <strong>and</strong> are quite sensitive to<br />
shock, thus giving rise to premature explosions which<br />
often result fatally. Trinitrotoluene is very inactive<br />
toward the greater number of the metals. Furthermore,<br />
picric acid has a high melting-point (122.5°)<br />
while the melting-point of trinitrotoluene is low enough<br />
(80.6°) that it may be poured into the shell in the<br />
molten state without danger from fire.