Reactions of Aromatic Compounds
Reactions of Aromatic Compounds
Reactions of Aromatic Compounds
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
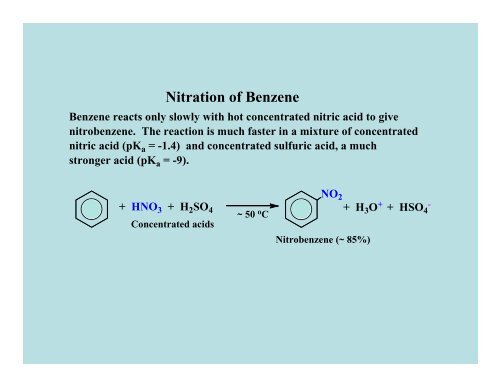
Nitration <strong>of</strong> Benzene<br />
Benzene reacts only slowly with hot concentrated nitric acid to give<br />
nitrobenzene. The reaction is much faster in a mixture <strong>of</strong> concentrated<br />
nitric acid (pK a = -1.4) and concentrated sulfuric acid, a much<br />
stronger acid (pK a = -9).<br />
+ HNO 3 + H 2SO 4<br />
Concentrated acids<br />
~ 50 o C<br />
NO 2<br />
+ H 3O + + HSO 4 -<br />
Nitrobenzene (~ 85%)