Reactions of Aromatic Compounds
Reactions of Aromatic Compounds
Reactions of Aromatic Compounds
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
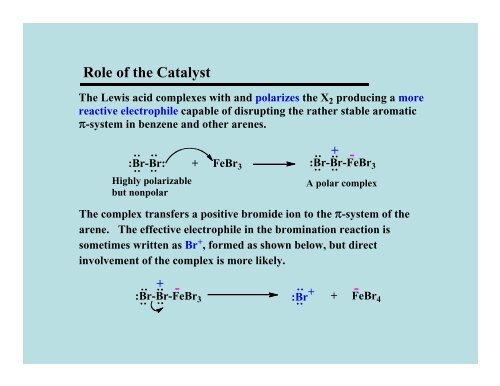
Role <strong>of</strong> the Catalyst<br />
The Lewis acid complexes with and polarizes the X 2 producing a more<br />
reactive electrophile capable <strong>of</strong> disrupting the rather stable aromatic<br />
π-system in benzene and other arenes.<br />
:Br-Br:<br />
: :<br />
: :<br />
Highly polarizable<br />
but nonpolar<br />
+ FeBr 3<br />
:Br-Br-FeBr 3<br />
: :<br />
+<br />
: :<br />
-<br />
A polar complex<br />
The complex transfers a positive bromide ion to the π-system <strong>of</strong> the<br />
arene. The effective electrophile in the bromination reaction is<br />
sometimes written as Br + , formed as shown below, but direct<br />
involvement <strong>of</strong> the complex is more likely.<br />
+<br />
:Br-Br-FeBr 3<br />
: :<br />
: :<br />
-<br />
:Br +<br />
: :<br />
-<br />
+ FeBr4