QM(DFT) and MD studies on formation mechanisms of C60 fullerenes
QM(DFT) and MD studies on formation mechanisms of C60 fullerenes
QM(DFT) and MD studies on formation mechanisms of C60 fullerenes
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
X Hua et al<br />
Figure 5. Energies calculated using the MSXX FF.<br />
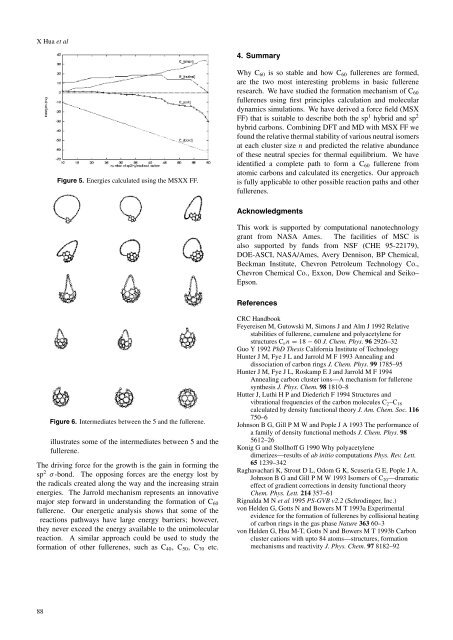
Figure 6. Intermediates between the 5 <str<strong>on</strong>g>and</str<strong>on</strong>g> the fullerene.<br />
illustrates some <strong>of</strong> the intermediates between 5 <str<strong>on</strong>g>and</str<strong>on</strong>g> the<br />
fullerene.<br />
The driving force for the growth is the gain in forming the<br />
sp 2 σ -b<strong>on</strong>d. The opposing forces are the energy lost by<br />
the radicals created al<strong>on</strong>g the way <str<strong>on</strong>g>and</str<strong>on</strong>g> the increasing strain<br />
energies. The Jarrold mechanism represents an innovative<br />
major step forward in underst<str<strong>on</strong>g>and</str<strong>on</strong>g>ing the formati<strong>on</strong> <strong>of</strong> <strong>C60</strong><br />
fullerene. Our energetic analysis shows that some <strong>of</strong> the<br />
reacti<strong>on</strong>s pathways have large energy barriers; however,<br />
they never exceed the energy available to the unimolecular<br />
reacti<strong>on</strong>. A similar approach could be used to study the<br />
formati<strong>on</strong> <strong>of</strong> other <strong>fullerenes</strong>, such as C40, C50, C70 etc.<br />
88<br />
4. Summary<br />
Why <strong>C60</strong> is so stable <str<strong>on</strong>g>and</str<strong>on</strong>g> how <strong>C60</strong> <strong>fullerenes</strong> are formed,<br />
are the two most interesting problems in basic fullerene<br />
research. We have studied the formati<strong>on</strong> mechanism <strong>of</strong> <strong>C60</strong><br />
<strong>fullerenes</strong> using first principles calculati<strong>on</strong> <str<strong>on</strong>g>and</str<strong>on</strong>g> molecular<br />
dynamics simulati<strong>on</strong>s. We have derived a force field (MSX<br />
FF) that is suitable to describe both the sp 1 hybrid <str<strong>on</strong>g>and</str<strong>on</strong>g> sp 2<br />
hybrid carb<strong>on</strong>s. Combining <str<strong>on</strong>g>DFT</str<strong>on</strong>g> <str<strong>on</strong>g>and</str<strong>on</strong>g> <str<strong>on</strong>g>MD</str<strong>on</strong>g> with MSX FF we<br />
found the relative thermal stability <strong>of</strong> various neutral isomers<br />
at each cluster size n <str<strong>on</strong>g>and</str<strong>on</strong>g> predicted the relative abundance<br />
<strong>of</strong> these neutral species for thermal equilibrium. We have<br />
identified a complete path to form a <strong>C60</strong> fullerene from<br />
atomic carb<strong>on</strong>s <str<strong>on</strong>g>and</str<strong>on</strong>g> calculated its energetics. Our approach<br />
is fully applicable to other possible reacti<strong>on</strong> paths <str<strong>on</strong>g>and</str<strong>on</strong>g> other<br />
<strong>fullerenes</strong>.<br />
Acknowledgments<br />
This work is supported by computati<strong>on</strong>al nanotechnology<br />
grant from NASA Ames. The facilities <strong>of</strong> MSC is<br />
also supported by funds from NSF (CHE 95-22179),<br />
DOE-ASCI, NASA/Ames, Avery Dennis<strong>on</strong>, BP Chemical,<br />
Beckman Institute, Chevr<strong>on</strong> Petroleum Technology Co.,<br />
Chevr<strong>on</strong> Chemical Co., Exx<strong>on</strong>, Dow Chemical <str<strong>on</strong>g>and</str<strong>on</strong>g> Seiko–<br />
Eps<strong>on</strong>.<br />
References<br />
CRC H<str<strong>on</strong>g>and</str<strong>on</strong>g>book<br />
Feyereisen M, Gutowski M, Sim<strong>on</strong>s J <str<strong>on</strong>g>and</str<strong>on</strong>g> Alm J 1992 Relative<br />
stabilities <strong>of</strong> fullerene, cumulene <str<strong>on</strong>g>and</str<strong>on</strong>g> polyacetylene for<br />
structures Cnn = 18 − 60 J. Chem. Phys. 96 2926–32<br />
Guo Y 1992 PhD Thesis California Institute <strong>of</strong> Technology<br />
Hunter J M, Fye J L <str<strong>on</strong>g>and</str<strong>on</strong>g> Jarrold M F 1993 Annealing <str<strong>on</strong>g>and</str<strong>on</strong>g><br />
dissociati<strong>on</strong> <strong>of</strong> carb<strong>on</strong> rings J. Chem. Phys. 99 1785–95<br />
Hunter J M, Fye J L, Roskamp E J <str<strong>on</strong>g>and</str<strong>on</strong>g> Jarrold M F 1994<br />
Annealing carb<strong>on</strong> cluster i<strong>on</strong>s—A mechanism for fullerene<br />
synthesis J. Phys. Chem. 98 1810–8<br />
Hutter J, Luthi H P <str<strong>on</strong>g>and</str<strong>on</strong>g> Diederich F 1994 Structures <str<strong>on</strong>g>and</str<strong>on</strong>g><br />
vibrati<strong>on</strong>al frequencies <strong>of</strong> the carb<strong>on</strong> molecules C2–C18<br />
calculated by density functi<strong>on</strong>al theory J. Am. Chem. Soc. 116<br />
750–6<br />
Johns<strong>on</strong> B G, Gill PMW<str<strong>on</strong>g>and</str<strong>on</strong>g>Pople J A 1993 The performance <strong>of</strong><br />
a family <strong>of</strong> density functi<strong>on</strong>al methods J. Chem. Phys. 98<br />
5612–26<br />
K<strong>on</strong>ig G <str<strong>on</strong>g>and</str<strong>on</strong>g> Stollh<strong>of</strong>f G 1990 Why polyacetylene<br />
dimerizes—results <strong>of</strong> ab initio computati<strong>on</strong>s Phys. Rev. Lett.<br />
65 1239–342<br />
Raghavachari K, Strout D L, Odom G K, Scuseria G E, Pople J A,<br />
Johns<strong>on</strong>BG<str<strong>on</strong>g>and</str<strong>on</strong>g>GillPMW1993 Isomers <strong>of</strong> C20—dramatic<br />
effect <strong>of</strong> gradient correcti<strong>on</strong>s in density functi<strong>on</strong>al theory<br />
Chem. Phys. Lett. 214 357–61<br />
Rignalda M N et al 1995 PS-GVB v2.2 (Schrodinger, Inc.)<br />
v<strong>on</strong> Helden G, Gotts N <str<strong>on</strong>g>and</str<strong>on</strong>g> Bowers M T 1993a Experimental<br />
evidence for the formati<strong>on</strong> <strong>of</strong> <strong>fullerenes</strong> by collisi<strong>on</strong>al heating<br />
<strong>of</strong> carb<strong>on</strong> rings in the gas phase Nature 363 60–3<br />
v<strong>on</strong> Helden G, Hsu M-T, Gotts N <str<strong>on</strong>g>and</str<strong>on</strong>g> Bowers M T 1993b Carb<strong>on</strong><br />
cluster cati<strong>on</strong>s with upto 84 atoms—structures, formati<strong>on</strong><br />
<strong>mechanisms</strong> <str<strong>on</strong>g>and</str<strong>on</strong>g> reactivity J. Phys. Chem. 97 8182–92