Laterite Leach Tests
Laterite Leach Tests
Laterite Leach Tests
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
ANSTO Minerals Report C1206 to Lagoon Creek Resources – Westmoreland Deposits<br />
4.2.4 Comparison of Oxidants<br />
For ease of control, sodium permanganate was used in most leach test. Base case leaches for<br />
Junnagunna and Redtree were also carried out using pyrolusite to demonstrate that both<br />
oxidants gave equivalent results. The base case leach data for the two oxidants are compared<br />
in Table 4.7. The oxidation equations for the two oxidants are given below, and show that<br />
differences in acid consumption for the Fe 2+ oxidation reaction need to be considered:<br />
4H + + MnO2 + 2Fe 2+ → Mn 2+ + 2Fe 3+ + 2H2O (1)<br />
8H + + MnO4 - + 5Fe 2+ → Mn 2+ + 5Fe 3+ + 4H2O (2)<br />
When pyrolusite is used as an oxidant, for every mole of Fe 2+ oxidised one mole of H2SO4 is<br />
consumed. As the ratio is 0.8 moles of H2SO4 consumed for every mole of Fe 2+ for<br />
permanganate, acid additions due to Fe 2+ oxidation are 20% lower when this oxidant is used.<br />
Thus for a sodium permanganate addition of 1.6 kg/t, the acid saving compared to MnO2<br />
would be 1.1 kg/t. As shown in Table 4.8, acid requirements for pyrolusite were in fact<br />
slightly less for pyrolusite after 24 h, but about 0.6 kg/t greater on average at 12 h when<br />
oxidant addition was stopped. For pyrolusite containing 75% reactive MnO2, the equivalent<br />
addition to 1.6 kg/t sodium permanganate is 3.3 kg/t. This predicted requirement is close to<br />
the experimental data.<br />
The results show that essentially the same extractions of uranium were obtained for the two<br />
oxidants after 24 h. Figure 4.18 also indicates that the rate of leaching was almost identical<br />
for the samples, but noting that the initial rate for pyrolusite with Junnagunna was slower than<br />
permanganate, probably resulting from the additional time to reach ORP set-point with<br />
pyrolusite at the start of the leach. (as pyrolusite reacts relatively slowly, care is taken not to<br />
overdose at the start of leaching).<br />
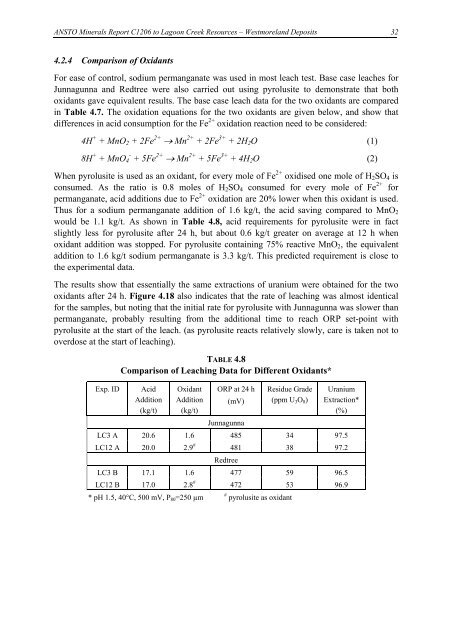
TABLE 4.8<br />
Comparison of <strong>Leach</strong>ing Data for Different Oxidants*<br />
Exp. ID Acid<br />
Addition<br />
(kg/t)<br />
Oxidant ORP at 24 h<br />
Addition (mV)<br />
(kg/t)<br />
Junnagunna<br />
Residue Grade<br />
(ppm U3O8)<br />
Uranium<br />
Extraction*<br />
(%)<br />
LC3 A 20.6 1.6 485 34 97.5<br />
LC12 A 20.0 2.9 # 481<br />
Redtree<br />
38 97.2<br />
LC3 B 17.1 1.6 477 59 96.5<br />
LC12 B 17.0 2.8 # 472 53 96.9<br />
* pH 1.5, 40°C, 500 mV, P80=250 µm # pyrolusite as oxidant<br />
32