Laterite Leach Tests
Laterite Leach Tests
Laterite Leach Tests
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
ANSTO Minerals Report C1206 to Lagoon Creek Resources – Westmoreland Deposits<br />
U 3O 8 in bulk eluate, mg/L<br />
12.0<br />
10.0<br />
8.0<br />
6.0<br />
4.0<br />
2.0<br />
0.0<br />
0 5 10 15 20<br />
V eluant (BV)<br />
Bulk eluate: Amberjet 4400 Bulk eluate: Ambersep 920<br />
Acid used: Amberjet 4400 Acid used: Ambersep 920<br />
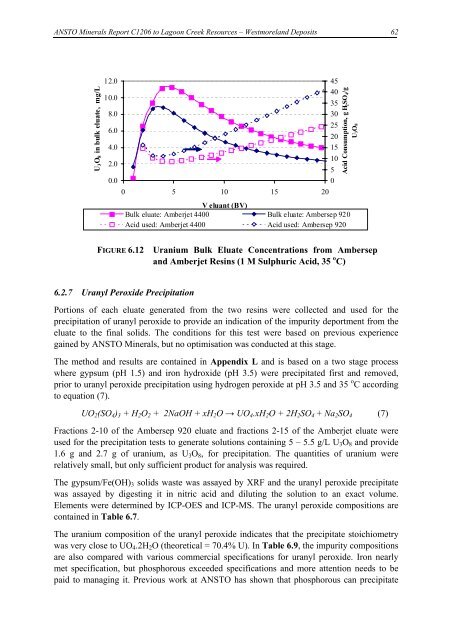
FIGURE 6.12 Uranium Bulk Eluate Concentrations from Ambersep<br />
and Amberjet Resins (1 M Sulphuric Acid, 35 o C)<br />
6.2.7 Uranyl Peroxide Precipitation<br />
Portions of each eluate generated from the two resins were collected and used for the<br />
precipitation of uranyl peroxide to provide an indication of the impurity deportment from the<br />
eluate to the final solids. The conditions for this test were based on previous experience<br />
gained by ANSTO Minerals, but no optimisation was conducted at this stage.<br />
The method and results are contained in Appendix L and is based on a two stage process<br />
where gypsum (pH 1.5) and iron hydroxide (pH 3.5) were precipitated first and removed,<br />
prior to uranyl peroxide precipitation using hydrogen peroxide at pH 3.5 and 35 o C according<br />
to equation (7).<br />
UO2(SO4)3 + H2O2 + 2NaOH + xH2O → UO4.xH2O + 2H2SO4 + Na2SO4 (7)<br />
Fractions 2-10 of the Ambersep 920 eluate and fractions 2-15 of the Amberjet eluate were<br />
used for the precipitation tests to generate solutions containing 5 – 5.5 g/L U3O8 and provide<br />
1.6 g and 2.7 g of uranium, as U3O8, for precipitation. The quantities of uranium were<br />
relatively small, but only sufficient product for analysis was required.<br />
The gypsum/Fe(OH)3 solids waste was assayed by XRF and the uranyl peroxide precipitate<br />
was assayed by digesting it in nitric acid and diluting the solution to an exact volume.<br />
Elements were determined by ICP-OES and ICP-MS. The uranyl peroxide compositions are<br />
contained in Table 6.7.<br />
The uranium composition of the uranyl peroxide indicates that the precipitate stoichiometry<br />
was very close to UO4.2H2O (theoretical = 70.4% U). In Table 6.9, the impurity compositions<br />
are also compared with various commercial specifications for uranyl peroxide. Iron nearly<br />
met specification, but phosphorous exceeded specifications and more attention needs to be<br />
paid to managing it. Previous work at ANSTO has shown that phosphorous can precipitate<br />
45<br />
40<br />
35<br />
30<br />
25<br />
20<br />
15<br />
10<br />
5<br />
0<br />
Acid Consumption, g H 2SO 4/g<br />
U3O8<br />
62