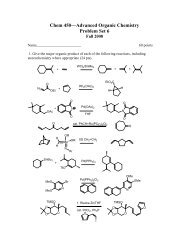
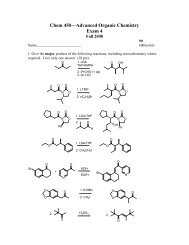
Amino Acids, Peptides, and Proteins
Amino Acids, Peptides, and Proteins
Amino Acids, Peptides, and Proteins
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Amino</strong> <strong>Acids</strong>, <strong>Peptides</strong>, <strong>and</strong> <strong>Proteins</strong> Introduction<br />
<strong>Amino</strong> <strong>Acids</strong><br />
<strong>Amino</strong> acids are the building blocks of proteins. In class you learned the structures of the 20<br />
common amino acids that make up proteins. All amino acids have the general structure shown<br />
below.<br />
H O<br />
H3N + C C<br />
R<br />
O -<br />
<strong>Peptides</strong><br />
<strong>Peptides</strong> are short chains of amino acids, each one connected to the next by an amide linkage<br />
called a peptide bond.<br />
Below is the chemical reaction by which two amino acids become connected by an amide<br />
linkage (the circled O <strong>and</strong> two H‟s are eliminated as a water molecule during the reaction):<br />
H<br />
O<br />
H 3N + C C<br />
R<br />
O -<br />
H<br />
H<br />
O<br />
N + + H C C<br />
H3N + H2O + C C N C C O -<br />
A tripeptide (composed of three amino acids):<br />
H O H O H O<br />
H 3N + C C<br />
R<br />
H<br />
N C C<br />
H<br />
R<br />
R<br />
two peptide bonds<br />
N<br />
H<br />
O -<br />
C C<br />
R<br />
O -<br />
H<br />
R<br />
O<br />
H<br />
H<br />
R<br />
peptide bond<br />
The artificial sweetener aspartame (br<strong>and</strong> name Nutrasweet) is an example of a modified<br />
peptide. The structure of aspartame is shown below.<br />
H<br />
H3N + C C<br />
CH2<br />
O<br />
C<br />
O O -<br />
N C C<br />
H<br />
H<br />
CH2<br />
O<br />
O<br />
CH3<br />
O<br />
1
<strong>Proteins</strong><br />
<strong>Proteins</strong> are the work horses of living cells. They act as microscopic cellular machines that<br />
function in much the same manner as human-built machines. Protein molecules are long chains<br />
of amino acids connected to each other in the same manner as in peptides. Very small proteins<br />
may be composed of 50 to 100 amino acids, while large proteins may contain thous<strong>and</strong>s of<br />
amino acids. In a normal functional protein, the long chain of amino acids is “folded” into a 3dimensional<br />
shape (imagine the long amino acid chain as a piece of string that has been<br />
crumpled up into a 3-D ball/blob). Every protein has a unique 3-dimensional shape that is suited<br />
to its biological function in a living organism. If the 3-dimensional shape “unfolds” for some<br />
reason, the protein will no longer be able to carry out its biological function <strong>and</strong> will usually be<br />
destroyed by the cell. (Unfolding is like the crumpled up blob of string being unwound again.)<br />
The unfolding of a protein structure is called denaturation.<br />
Chemical Tests<br />
In this experiment you will perform three chemical tests to distinguish between free amino acids,<br />
peptides, <strong>and</strong> proteins in the lab.<br />
Biuret Test<br />
The Biuret reagent contains copper ions which give it a blue color. The copper ions will interact<br />
with a compound that contains two or more peptide bonds, resulting in the formation of a<br />
violet/purple-colored product. When a compound does not have at least two peptide bonds, it<br />
will not react with the Biuret reagent, <strong>and</strong> no purple color will appear (solution will remain a<br />
shade of blue due to the copper ions).<br />
Positive Biuret test: violet/purple product forms<br />
Negative Biuret test: no violet/purple product formed<br />
Ninhydrin Test<br />
The ninhydrin reagent will react specifically with a primary (1 o ) amino functional group on a<br />
compound, resulting in the formation of a violet/purple-colored product. When a compound does<br />
not have a primary amino group, it will not react with the ninhydrin reagent, <strong>and</strong> no purple color<br />
will appear (solution will remain colorless).<br />
Positive ninhydrin test: violet/purple product forms<br />
Negative ninhydrin test: no violet/purple product formed<br />
Denaturation Test<br />
Strong acid will often denature (unfold) proteins. <strong>Proteins</strong> that are properly folded into their<br />
normal 3-dimensional shape tend to be soluble in aqueous solution. However, proteins that<br />
have been denatured tend to clump together <strong>and</strong> come out of solution as a precipitate because<br />
they are no longer soluble when they are denatured <strong>and</strong> clumped together. In the denaturation<br />
test, strong acid is used to test a solution for the presence of protein. When strong acid is<br />
added, the formation of a white precipitate (composed of denatured protein molecules) is<br />
considered a positive denaturation test <strong>and</strong> indicates the presence of protein in the solution. If<br />
no precipitate forms, the denaturation test is negative, <strong>and</strong> indicates that no protein is present in<br />
the solution.<br />
2
<strong>Amino</strong> <strong>Acids</strong>, <strong>Peptides</strong>, <strong>and</strong> <strong>Proteins</strong> Prelab Name_______________<br />
1. Draw the structures of alanine <strong>and</strong> glycine. (Refer to the figure showing the 20 amino acids<br />
which is included in your In-Class Biochemistry Notebook.)<br />
2. Show the structure of the peptide that would result from a chemical reaction between glycine<br />
<strong>and</strong> alanine.<br />
3. A. Indicate whether each of the following is an amino acid, a peptide, or a protein.<br />
B. Indicate whether each will give a positive or negative Biuret test.<br />
A. aa, pept, prot? B. Biuret test? (+ or - )<br />
aspartic acid (a.k.a. aspartate) ____________ ___________<br />
phenylalanine ____________ ___________<br />
Nutrasweet (aspartame) ____________ ___________<br />
4. Examine the structure of aspartame (Nutrasweet) in the introduction, paying careful attention<br />
to the side chains. Which two amino acids would be released if the peptide bond was<br />
„hydrolyzed‟ (broken by a reaction with H2O)?<br />
3
<strong>Amino</strong> <strong>Acids</strong>, <strong>Peptides</strong>, <strong>and</strong> <strong>Proteins</strong> Procedure<br />
Isolation of the Protein Casein from Milk<br />
1. Put 50 mL of skim milk into a 250 mL beaker. While stirring gently, add a solution of 10%<br />
acetic acid dropwise to the milk. As the mixture becomes acidic (at approximately pH 5.5), the<br />
milk protein casein becomes insoluble <strong>and</strong> begins to precipitate. Add acetic acid until you see<br />
no further precipitation forming.<br />
2. Prepare a simple filtration apparatus as follows. Place a square of cheese cloth (about 3<br />
layers thick) over the top of a large beaker—the cheesecloth square should be just large enough<br />
to cover the top of the beaker with a little overlap at the edges. Secure the cheesecloth using a<br />
rubber b<strong>and</strong>.<br />
3. Pour your precipitated casein mixture through the cheesecloth. Wash the casein with 25 mL<br />
of ethanol. Scrape the casein off the cheesecloth <strong>and</strong> press it between some paper towels to<br />
dry it.<br />
4. Take about half of your casein <strong>and</strong> put it in a 50 mL beaker. Add 10 mL of 0.1 M NaOH to<br />
the casein <strong>and</strong> stir to dissolve. This is the solution of dissolved casein you will use in the<br />
following steps.<br />
Biuret Test<br />
1. Use the dilute soap solution in the bottles near the sinks (bottles with blue lids) to thoroughly<br />
clean 5 test tubes. Rinse them well with distilled water. It is very important that the test tubes be<br />
clean in order to prevent anomalous results in the chemical tests you will carry out. The test<br />
tubes do not have to be completely dry, but shake them to remove as much water as possible.<br />
2. NOTE: glycylglycine is a dipeptide composed of two glycine‟s linked together.<br />
Place the following chemicals into four test tubes as indicated:<br />
Tube 1: 1 mL distilled water (water will serve as a control)<br />
Tube 2: 1 mL 1% alanine solution<br />
Tube 3: 1mL 1% glycylglycine solution<br />
Tube 4: 5 drops of your casein solution (from step 4 above) + 1 mL distilled water<br />
NOTE: It may take more than 5 drops of the casein. Start with 5 drops <strong>and</strong><br />
show your results to the instructor to see if this is enough.<br />
Tube 5: 1 mL of the unknown<br />
Then add 1 mL of the biuret solution to each of the five test tubes. Allow the contents to st<strong>and</strong><br />
for 10 minutes. While waiting, fill a 250 mL beaker about 1/3 full with distilled water <strong>and</strong> place it<br />
on a hot plate, but don‟t turn on the hot plate yet (you‟ll need it for the ninhydrin test below). If<br />
you wish, you may also do step 1 of the ninhydrin test below while you are waiting.<br />
4
3. At the end of 10 minutes, observe <strong>and</strong> record the color of the test tube contents on the report<br />
sheet. Answer the questions regarding interpretation of the test results. The contents of the test<br />
tubes can then be discarded down the drain.<br />
Ninhydrin Test<br />
1. Your casein solution is acidic (pH below 7), but it needs to be neutral (pH ≈ 7) to conduct a<br />
ninhydrin test. Neutralize your casein solution as follows. Put 3 drops of the casein solution<br />
from step 4 above into a clean test tube <strong>and</strong> add 1 mL of distilled water.<br />
Test the pH of the solution by dipping a glass stirring rod into the solution <strong>and</strong> then touching the<br />
end of the rod onto a piece of pH paper.<br />
If the pH is too high (above 7-8), add a drop of 0.3 M HCl to the test tube, mix the<br />
contents of the tube, <strong>and</strong> test the pH again.<br />
If the pH is too low (below 7-8), add a drop of 0.3 M NaOH to the test tube, mix the<br />
contents of the tube, <strong>and</strong> test the pH again.<br />
Use more drops of HCl or NaOH as needed to achieve a pH of 7 to 8.<br />
2. Turn the hot plate on high to start heating the water—you will need boiling water for the<br />
ninhydrin test.<br />
3. Wash the five test tubes from the Biuret test thoroughly so they can be re-used now. They<br />
need not be completely dry. Place the following chemicals into the five clean test tubes:<br />
Tube 1: 1 mL distilled water (water will serve as a control)<br />
Tube 2: 1 mL 1% alanine solution<br />
Tube 3: 1mL 1% glycylglycine solution<br />
Tube 4: 1 mL of neutralized casein solution from step 1 directly above<br />
Tube 5: 1 mL of the unknown<br />
Then add 1 mL of 0.2% ninhydrin solution to each test tube. Label the tubes clearly using a<br />
grease pencil. Don‟t use tape to label the tubes because the tape will fall off in the boiling water<br />
bath in the next step.<br />
4. Once the water is boiling moderately, place the tubes in the boiling water bath for 5<br />
minutes—be sure that the water level is not so high that water boils over into the test tubes.<br />
At the end of 5 minutes, turn off the hot plate. Observe <strong>and</strong> record the color of the tube contents<br />
on the report sheet. Answer the questions regarding interpretation of the test results. The<br />
contents of the test tubes can then be discarded down the drain.<br />
5
Denaturation with Acid<br />
1. Wash the five test tubes from the ninhydrin test thoroughly so they can be re-used now. Be<br />
sure to use a test tube brush <strong>and</strong> soap in order to remove any purple residue from the ninhydrin<br />
test. The tubes need not be completely dry. Place the following chemicals into the five clean<br />
test tubes:<br />
Tube 1: 1 mL distilled water (water will serve as a control)<br />
Tube 2: 1 mL 1% alanine solution<br />
Tube 3: 1mL 1% glycylglycine solution<br />
Tube 4: 10 drops of your solution of dissolved casein in the 50 mL beaker from step<br />
4 under “Isolation of the Protein Casein from Milk”<br />
Tube 5: 1 mL of the unknown<br />
Then add 1 mL of 20% trichloroacetic acid to each tube. Mix the contents of the tubes by flicking<br />
them with your finger. NOTE: if you see purple color in any of the solutions, the test tube was<br />
not thoroughly cleaned after the ninhydrin test.<br />
2. Observe <strong>and</strong> record your observations on the report sheet. (There may be nothing to<br />
observe—no changes—in some of the tubes.) Answer the questions regarding interpretation of<br />
the test results. The contents of the test tubes can then be discarded down the drain.<br />
3. All other solutions can be discarded down the drain. The solid casein can be discarded in the<br />
trash.<br />
6
<strong>Amino</strong> <strong>Acids</strong>, <strong>Peptides</strong>, <strong>and</strong> <strong>Proteins</strong> Report Sheet Name ___________<br />
Biuret Test Color observed<br />
water (control)<br />
1% alanine<br />
1% glycylglycine<br />
casein<br />
unknown<br />
Interpretation of Biuret Test Results<br />
It will be helpful to review the structures of alanine <strong>and</strong> glycylglycine (see Postlab #1, #2) in<br />
order to interpret the results of the test.<br />
What do the results of the Biuret test tell you about alanine?<br />
What do the results of the Biuret test tell you about glycylglycine?<br />
What do the results of the Biuret test tell you about casein?<br />
Ninhydrin Test Color observed<br />
water (control)<br />
1% alanine<br />
1% glycylglycine<br />
casein<br />
unknown<br />
7
<strong>Amino</strong> <strong>Acids</strong>, <strong>Peptides</strong>, <strong>and</strong> <strong>Proteins</strong> Report Sheet Name ___________<br />
Interpretation of Ninhydrin Test Results<br />
It will be helpful to review the structures of alanine <strong>and</strong> glycylglycine (see Postlab #1, #2) in<br />
order to interpret the results of the test.<br />
What do the results of the ninhydrin test tell you about alanine?<br />
What do the results of the ninhydrin test tell you about glycylglycine?<br />
What do the results of the ninhydrin test tell you about casein?<br />
Denaturation Test Observations<br />
water (control)<br />
1% alanine<br />
1% glycylglycine<br />
casein<br />
unknown<br />
Interpretation of Denaturation Test Results<br />
What do the results of the denaturation test tell you about alanine?<br />
What do the results of the denaturation test tell you about glycylglycine?<br />
What do the results of the denaturation test tell you about casein?<br />
8
<strong>Amino</strong> <strong>Acids</strong>, <strong>Peptides</strong>, <strong>and</strong> <strong>Proteins</strong> Postlab Name_______________<br />
1. Draw the structure of glycylglycine (first refer to the “NOTE” on step 2 on page 4). Use an<br />
arrow <strong>and</strong> a brief description to indicate the structural feature that caused the glycylglycine<br />
solution to produce the Biuret test result that it did.<br />
2. Draw the structure of alanine. Circle the structural feature that caused the alanine solution to<br />
produce the ninhydrin test result that it did.<br />
3. Look at the structure of aspartame (Nutrasweet) in the introduction. Predict the results<br />
(+ or -) of the following tests on aspartame:<br />
Biuret test:___ Ninhydrin test:___ Denaturation test:___<br />
4. Oxytocin is a hormone; it is a 9-amino acid peptide. Predict the results (+ or -) of the<br />
following tests on oxytocin:<br />
Biuret test:___ Ninhydrin test:___ Denaturation test:___<br />
5. Hemoglobin is a protein composed of over 100 amino acids linked together by peptide bonds.<br />
Predict the results (+ or -) of the following tests on hemoglobin:<br />
Biuret test:___ Ninhydrin test:___ Denaturation test:___<br />
6. Look back at the test results you obtained for the uknown. Based on the test results, check<br />
the identity of your unknown.<br />
An amino acid ___<br />
A pentapeptide (composed of 5 amino acids) ___<br />
A protein (composed of several hundred amino acids) ___<br />
7. What was the purpose of having water as a control for each test?<br />
8. You have a test tube containing either a dipeptide or a large protein. What tests could you<br />
use to determine which is in the test tube?<br />
9