Ph.D. THESIS ABSTRACT SYNTHESIS, STEREOCHEMISTRY AND ...
Ph.D. THESIS ABSTRACT SYNTHESIS, STEREOCHEMISTRY AND ...
Ph.D. THESIS ABSTRACT SYNTHESIS, STEREOCHEMISTRY AND ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Radu Gropeanu – <strong>Ph</strong>D Thesis Abstract<br />
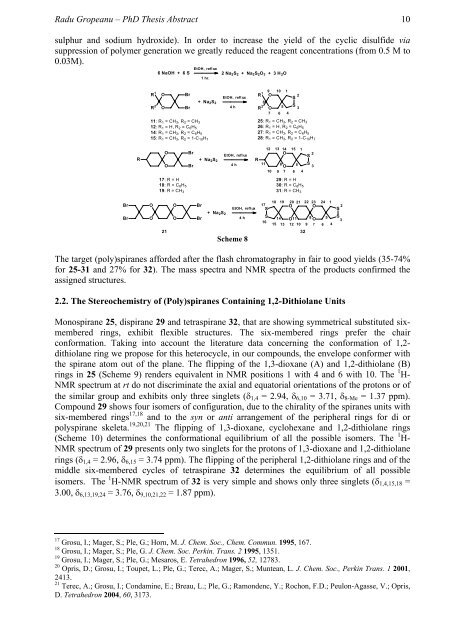
sulphur and sodium hydroxide). In order to increase the yield of the cyclic disulfide via<br />
suppression of polymer generation we greatly reduced the reagent concentrations (from 0.5 M to<br />
0.03M).<br />
Br<br />
Br<br />
R 1<br />
R 2<br />
EtOH, reflux 6 NaOH + 6 S 2 Na2S2 + Na2S2O3 + 3 H2O<br />
1 hr.<br />
O<br />
O<br />
Br<br />
Br<br />
+ Na2S2<br />
11: R1 = CH3, R2 = CH3<br />
12: R 1 = H, R 2 = C 6H 5<br />
14: R 1 = CH 3, R 2 = C 6H 5<br />
15: R 1 = CH 3, R 2 = 1-C 10H 7<br />
EtOH, reflux R O<br />
4 h O<br />
1<br />
R 2<br />
9 10 1<br />
2<br />
S<br />
8<br />
5 S<br />
3<br />
7 6 4<br />
25: R1 = CH3, R2 = CH3<br />
26: R 1 = H, R 2 = C 6H 5<br />
27: R 1 = CH 3, R 2 = C 6H 5<br />
28: R1 = CH3, R2 = 1-C10H7<br />
R<br />
O<br />
O<br />
Br<br />
Br<br />
+ Na2S2 EtOH, reflux 4 h<br />
12<br />
R<br />
11<br />
13 14<br />
O<br />
8<br />
O<br />
15 1<br />
5<br />
2<br />
S<br />
S<br />
3<br />
10 9 7 6 4<br />
O<br />
17: R = H<br />
18: R = C 6H 5<br />
19: R = CH 3<br />
O<br />
O O<br />
Br<br />
Br<br />
+ Na2S2<br />
29: R = H<br />
30: R = C 6H 5<br />
31: R = CH 3<br />
EtOH, refl ux<br />
17<br />
S<br />
18 19 20 21<br />
O<br />
22 23<br />
O<br />
24 1<br />
2<br />
S<br />
4 h S<br />
16<br />
14<br />
15 13<br />
O11<br />
12 10<br />
8 O<br />
9 7<br />
5<br />
6 4<br />
S<br />
3<br />
21 32<br />
Scheme 8<br />
The target (poly)spiranes afforded after the flash chromatography in fair to good yields (35-74%<br />
for 25-31 and 27% for 32). The mass spectra and NMR spectra of the products confirmed the<br />
assigned structures.<br />
2.2. The Stereochemistry of (Poly)spiranes Containing 1,2-Dithiolane Units<br />
Monospirane 25, dispirane 29 and tetraspirane 32, that are showing symmetrical substituted sixmembered<br />
rings, exhibit flexible structures. The six-membered rings prefer the chair<br />
conformation. Taking into account the literature data concerning the conformation of 1,2dithiolane<br />
ring we propose for this heterocycle, in our compounds, the envelope conformer with<br />
the spirane atom out of the plane. The flipping of the 1,3-dioxane (A) and 1,2-dithiolane (B)<br />
rings in 25 (Scheme 9) renders equivalent in NMR positions 1 with 4 and 6 with 10. The 1 H-<br />
NMR spectrum at rt do not discriminate the axial and equatorial orientations of the protons or of<br />
the similar group and exhibits only three singlets (δ1,4 = 2.94, δ6,10 = 3.71, δ8-Me = 1.37 ppm).<br />
Compound 29 shows four isomers of configuration, due to the chirality of the spiranes units with<br />
six-membered rings 17,18 and to the syn or anti arrangement of the peripheral rings for di or<br />
polyspirane skeleta. 19,20,21 The flipping of 1,3-dioxane, cyclohexane and 1,2-dithiolane rings<br />
(Scheme 10) determines the conformational equilibrium of all the possible isomers. The 1 H-<br />
NMR spectrum of 29 presents only two singlets for the protons of 1,3-dioxane and 1,2-dithiolane<br />
rings (δ1,4 = 2.96, δ6,15 = 3.74 ppm). The flipping of the peripheral 1,2-dithiolane rings and of the<br />
middle six-membered cycles of tetraspirane 32 determines the equilibrium of all possible<br />
isomers. The 1 H-NMR spectrum of 32 is very simple and shows only three singlets (δ1,4,15,18 =<br />
3.00, δ6,13,19,24 = 3.76, δ9,10,21,22 = 1.87 ppm).<br />
17<br />
Grosu, I.; Mager, S.; Ple, G.; Horn, M. J. Chem. Soc., Chem. Commun. 1995, 167.<br />
18<br />
Grosu, I.; Mager, S.; Ple, G. J. Chem. Soc. Perkin. Trans. 2 1995, 1351.<br />
19<br />
Grosu, I.; Mager, S.; Ple, G.; Mesaros, E. Tetrahedron 1996, 52, 12783.<br />
20<br />
Opris, D.; Grosu, I.; Toupet, L.; Ple, G.; Terec, A.; Mager, S.; Muntean, L. J. Chem. Soc., Perkin Trans. 1 2001,<br />
2413.<br />
21<br />
Terec, A.; Grosu, I.; Condamine, E.; Breau, L.; Ple, G.; Ramondenc, Y.; Rochon, F.D.; Peulon-Agasse, V.; Opris,<br />
D. Tetrahedron 2004, 60, 3173.<br />
10