Ph.D. THESIS ABSTRACT SYNTHESIS, STEREOCHEMISTRY AND ...
Ph.D. THESIS ABSTRACT SYNTHESIS, STEREOCHEMISTRY AND ...
Ph.D. THESIS ABSTRACT SYNTHESIS, STEREOCHEMISTRY AND ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Radu Gropeanu – <strong>Ph</strong>D Thesis Abstract<br />
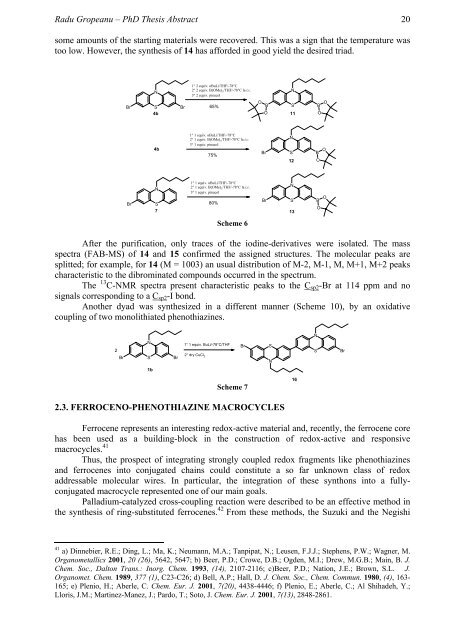
some amounts of the starting materials were recovered. This was a sign that the temperature was<br />
too low. However, the synthesis of 14 has afforded in good yield the desired triad.<br />
N<br />
1° 2 equiv. nBuLi/THF/-78°C<br />
2° 2 equiv. B(OMe) 3 /THF/-78°C la t.c.<br />
3° 2 equiv. pinacol<br />
N<br />
Br S<br />
Br<br />
65%<br />
O<br />
B S<br />
B<br />
O<br />
4b O 11 O<br />
Br<br />
4b<br />
N<br />
S<br />
7<br />
1° 1 equiv. nBuLi/THF/-78°C<br />
2° 1 equiv. B(OMe) 3 /THF/-78°C la t.c.<br />
3° 1 equiv. pinacol<br />
75%<br />
1° 1 equiv. nBuLi/THF/-78°C<br />
2° 1 equiv. B(OMe) 3 /THF/-78°C la t.c.<br />
3° 1 equiv. pinacol<br />
80%<br />
Scheme 6<br />
N<br />
Br S<br />
B<br />
O<br />
12 O<br />
N<br />
O<br />
Br S<br />
B<br />
After the purification, only traces of the iodine-derivatives were isolated. The mass<br />
spectra (FAB-MS) of 14 and 15 confirmed the assigned structures. The molecular peaks are<br />
splitted; for example, for 14 (M = 1003) an usual distribution of M-2, M-1, M, M+1, M+2 peaks<br />
characteristic to the dibrominated compounds occurred in the spectrum.<br />
The 13 C-NMR spectra present characteristic peaks to the Csp2-Br at 114 ppm and no<br />
signals corresponding to a Csp2-I bond.<br />
Another dyad was synthesized in a different manner (Scheme 10), by an oxidative<br />
coupling of two monolithiated phenothiazines.<br />
N<br />
2<br />
Br S<br />
Br<br />
1b<br />
1° 1 equiv. BuLi/-78°C/THF<br />
2° dry CuCl 2<br />
Br<br />
Scheme 7<br />
2.3. FERROCENO-PHENOTHIAZINE MACROCYCLES<br />
Ferrocene represents an interesting redox-active material and, recently, the ferrocene core<br />
has been used as a building-block in the construction of redox-active and responsive<br />
macrocycles. 41<br />
Thus, the prospect of integrating strongly coupled redox fragments like phenothiazines<br />
and ferrocenes into conjugated chains could constitute a so far unknown class of redox<br />
addressable molecular wires. In particular, the integration of these synthons into a fullyconjugated<br />
macrocycle represented one of our main goals.<br />
Palladium-catalyzed cross-coupling reaction were described to be an effective method in<br />
the synthesis of ring-substituted ferrocenes. 42 From these methods, the Suzuki and the Negishi<br />
41 a) Dinnebier, R.E.; Ding, L.; Ma, K.; Neumann, M.A.; Tanpipat, N.; Leusen, F.J.J.; Stephens, P.W.; Wagner, M.<br />
Organometallics 2001, 20 (26), 5642, 5647; b) Beer, P.D.; Crowe, D.B.; Ogden, M.I.; Drew, M.G.B.; Main, B. J.<br />
Chem. Soc., Dalton Trans.: Inorg. Chem. 1993, (14), 2107-2116; c)Beer, P.D.; Nation, J.E.; Brown, S.L. J.<br />
Organomet. Chem. 1989, 377 (1), C23-C26; d) Bell, A.P.; Hall, D. J. Chem. Soc., Chem. Commun. 1980, (4), 163-<br />
165; e) Plenio, H.; Aberle, C. Chem. Eur. J. 2001, 7(20), 4438-4446; f) Plenio, E.; Aberle, C.; Al Shihadeh, Y.;<br />
Lloris, J.M.; Martinez-Manez, J.; Pardo, T.; Soto, J. Chem. Eur. J. 2001, 7(13), 2848-2861.<br />
S<br />
N<br />
13<br />
16<br />
N<br />
S<br />
O<br />
Br<br />
20