Classwork 5.pdf - LMC
Classwork 5.pdf - LMC
Classwork 5.pdf - LMC
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
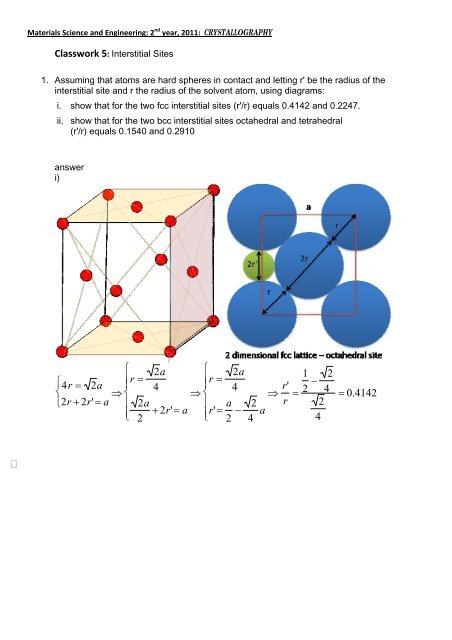
Materials Science and Engineering: 2 nd year, 2011: CRYSTALLOGRAPHY<br />
<strong>Classwork</strong> 5: Interstitial Sites<br />
1. Assuming that atoms are hard spheres in contact and letting r' be the radius of the<br />
interstitial site and r the radius of the solvent atom, using diagrams:<br />
i. show that for the two fcc interstitial sites (r'/r) equals 0.4142 and 0.2247.<br />
ii. show that for the two bcc interstitial sites octahedral and tetrahedral<br />
(r'/r) equals 0.1540 and 0.2910<br />
answer<br />
i)<br />
<br />
r <br />
4r 2a<br />
<br />
2r<br />
2r' a<br />
2a <br />
<br />
<br />
r <br />
4<br />
<br />
<br />
2a<br />
2r' a<br />
<br />
2 2a<br />
4<br />
r' a 2<br />
<br />
2 4 a<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
r'<br />
r <br />
1 2<br />
<br />
2 4<br />
2<br />
4<br />
0.4142
The tetrahedral interstice from the figure are situated at 1/4 of the diagonal. The distance<br />
between the center of a "corner" atom and the center of the tetrahedral interstice is: r+r'<br />
So to express the whole diagonal as a function of r and r' we have to multiply the previous<br />
distance by 4. Therefore we have the following system:<br />
4(r r') 3a<br />
r 2<br />
4 a<br />
<br />
<br />
<br />
<br />
<br />
ii)<br />
r'<br />
r<br />
(as before)<br />
3 2<br />
2<br />
0.2247<br />
<br />
r <br />
4r 3a<br />
<br />
2r<br />
2r' a<br />
3a <br />
<br />
<br />
r <br />
4<br />
<br />
<br />
2a<br />
2r' a<br />
<br />
2 3a<br />
4<br />
r' a 2<br />
<br />
2 4 a<br />
<br />
<br />
<br />
<br />
<br />
<br />
<br />
r'<br />
r <br />
1 3<br />
<br />
2 4<br />
3<br />
4<br />
0.1547
In the body centered cubic system the tetrahedral sites are located at 1/2 0 1/4.<br />
Therefore we can draw the following scheme:<br />
Using Pythagoras theorem we have the following system of equations:<br />
4r 3a<br />
(r r') 2 1<br />
4 a<br />
2<br />
<br />
<br />
<br />
1<br />
2 a<br />
<br />
r <br />
<br />
<br />
2<br />
<br />
<br />
<br />
<br />
<br />
3a <br />
<br />
4<br />
<br />
5 <br />
<br />
r' a<br />
r<br />
<br />
4 <br />
r'<br />
r<br />
5 3<br />
3<br />
0.2910
2. At the transition in pure iron at 910°C, the lattice constant changes from,<br />
a = 2.90 Å (bcc) to a = 3.64 Å (fcc).<br />
i. Show that for both crystal forms and both sites, the carbon atom (atomic radius = 0.77<br />
Å) distorts the lattice (i.e. it is larger than the site in which it resides).<br />
ii. Carbon atoms in a-iron (bcc) occupy the octahedral interstices; why?<br />
(hint consider the forces acting on the atoms and the symmetry of the two sites).<br />
Answer<br />
i) Consider the fcc lattice where a = 3.64 Å.<br />
We know that th carbon atom resides in the interstitial sites, therefore r' = 0.77 Å.<br />
For a fcc lattice we have the following relationship:<br />
4 √2 from classwork 4<br />
3.64 ∗ 1.414<br />
4 1.29<br />
Which means that: ´ .<br />
0.5969<br />
.<br />
We notice that 0.5969 > 0.4142 > 0.2247, explains why the carbon atoms are preferably in the<br />
octahedral sites (0.4142) because they are much larger and distort less the lattice than if they<br />
were in the tetrahedral sites.<br />
Consider now the bcc lattice where a = 2.90 Å and r' = 0.77 Å.<br />
4 √3 from classwork 4<br />
2.90 ∗ 1.732<br />
1.256<br />
4<br />
Which gives us: ´ .<br />
0.6132<br />
.<br />
Once again, we have 0.6132 > 0.2910 (tetrahedral) > 0.1547 (octahedral) which proves that<br />
the carbon atoms distort the lattice. Note that the tetrahedral sites are larger than octahedral<br />
ones.<br />
ii) Considering the symmetry part of the octahedral sites we can see that the carbon interstitial<br />
atoms have only 2 atoms to distort whereas in the tetrahedral sites they have 4 atoms making<br />
it more difficult.
3. Write down the coordinates of all the tetrahedral and octahedral sites for the fcc and bcc<br />
structures.<br />
Fcc - octahedral sites:<br />
(½ 0 0) (0 0 ½) (0 ½ 0)<br />
(½ ½ ½).<br />
Fcc - tetrahedral sites:<br />
(¼ ¼ ¼)<br />
(¾ ¼ ¼) (¼ ¾ ¼) (¼ ¼ ¾)<br />
(¾ ¾ ¼) (¾ ¼ ¾) (¼ ¾ ¾)<br />
(¾ ¾ ¾)<br />
Bcc - octahedral sites:<br />
(0 0 ½) (½ 0 0) (0 ½ 0)<br />
(½ 0 ½) (0 ½ ½) (½ ½ 0)<br />
Bcc - tetrahedral sites:<br />
(½ 0 ¼) (¼ 0 ½) (0 ½ ¼) (0 ¼ ½) (½ ¼ 0) (¼ ½ 0)<br />
(0 ½ ¾) (0 ¾ ½) (½ 0 ¾) (¾ 0 ½) (½ ¾ 0) (¾ ½ 0)