Investigation Manual for the Regulated Chemical Substances - Omron
Investigation Manual for the Regulated Chemical Substances - Omron
Investigation Manual for the Regulated Chemical Substances - Omron
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
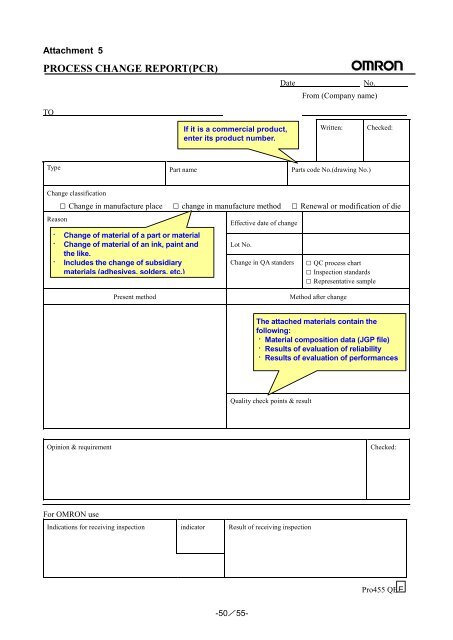
Attachment 5<br />
PROCESS CHANGE REPORT(PCR)<br />
TO<br />
Type<br />
Part name<br />
-50/55-<br />
Date No.<br />
From (Company name)<br />
Written: Checked:<br />
Parts code No.(drawing No.)<br />
Change classification<br />
□ Change in manufacture place □ change in manufacture method □ Renewal or modification of die<br />
Reason<br />
· Change of material of a part or material<br />
· Change of material of an ink, paint and<br />
<strong>the</strong> like.<br />
· Includes <strong>the</strong> change of subsidiary<br />
materials (adhesives, solders, etc.)<br />
Effective date of change<br />
Lot No.<br />
Change in QA standers<br />
□ QC process chart<br />
□ Inspection standards<br />
□ Representative sample<br />
Present method Method after change<br />
Quality check points & result<br />
Opinion & requirement Checked:<br />
For OMRON use<br />
If it is a commercial product,<br />
enter its product number.<br />
Indications <strong>for</strong> receiving inspection indicator Result of receiving inspection<br />
The attached materials contain <strong>the</strong><br />
following:<br />
· Material composition data (JGP file)<br />
· Results of evaluation of reliability<br />
· Results of evaluation of per<strong>for</strong>mances<br />
Pro455 QE E