Fellow name: Jennifer Casey Title of Lesson: Flame Test Activity ...
Fellow name: Jennifer Casey Title of Lesson: Flame Test Activity ...
Fellow name: Jennifer Casey Title of Lesson: Flame Test Activity ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Fellow</strong> <strong>name</strong>: <strong>Jennifer</strong> <strong>Casey</strong> <br />
<br />
<strong>Title</strong> <strong>of</strong> <strong>Lesson</strong>: <strong>Flame</strong> <strong>Test</strong> <strong>Activity</strong> <br />
<br />
Grade Level: 10 th , 11 th , and 12 th <br />
<br />
Subject(s): Chemistry and Honors Chemistry <br />
<br />
<br />
<br />
Summary: This lesson is centered on performing various flame tests for students in order for<br />
students to find periodic trends within the color changes observed. In order for students to<br />
understand why the color changes occur, the wave-like nature <strong>of</strong> light and the concept <strong>of</strong> electron<br />
excitation are reviewed first. The review is given through both a PowerPoint presentation and a<br />
handout for students. The handout includes pre-questions that help the teacher gauge the prior<br />
knowledge <strong>of</strong> students and a section <strong>of</strong> fill-in-the-blank notes that the students can answer during<br />
the presentation. Once these concepts have been thoroughly reviewed, the flame test demonstration<br />
can take place. Following this, there is a second handout that guides students through determining<br />
the periodic trends in color emitted. Lastly, this lesson is concluded with an at-home assignment<br />
where students can apply their knowledge <strong>of</strong> flame testing to two real world systems (chemical<br />
identification and astronomy). <br />
<br />
Time Required: This whole lesson can be completed in a 50‐minute class period. The <br />
presentation and first handout take no more than 25 minutes to complete, the flame test <br />
(which consists <strong>of</strong> testing seven compounds) takes about 10 minutes to complete, and the <br />
second worksheet, which helps students establish the periodic trends associated with flame <br />
testing, can easily be finished in the remaining 15 minutes. <br />
<br />
Group Size: There are no groups required for this activity. <br />
<br />
Cost to implement: The costs to implement this lesson are very low, as the salts needed in <br />
this demonstration are salts that are readily available in most school stock rooms. If a salt is <br />
unavailable, then that salt does not need to be included, and a different chloride salt can be <br />
used in its place. Also, the amount <strong>of</strong> salt needed is very little – the flame test requires only <br />
about half‐a‐gram to a gram <strong>of</strong> salt. <br />
<br />
Learning Goals: <br />
After this lesson, students should be able to:
1. Distinguish which color <strong>of</strong> light has the highest/lowest frequency <br />
2. Comment on light as a form <strong>of</strong> energy <br />
3. Understand what the world “excited” means in relation to electrons in an atom <br />
4. Identify a ground state from an excited state electron configuration <br />
5. Recognize that energy absorbed from atoms can be in the form <strong>of</strong> heat <br />
6. Recognize that energy emitted from atoms can be in the form <strong>of</strong> light <br />
7. Relate the idea <strong>of</strong> light emission to chemical identification <br />
<br />
Introduction / Motivation: <br />
**It should first be noted that this lesson relies heavily on the fact that students are <br />
comfortable with ground state electron configurations. They do not need to have learned <br />
about excited state configurations, but the primary goal <strong>of</strong> this lesson is to take something less <br />
interesting like electron configurations and show how it explains very interesting phenomena. <br />
So in that regards, this lesson is less about teaching a specific idea, and more about relating an <br />
already understood idea to various applications.** <br />
<br />
To introduce the material, I start out with a few simple examples to get students interested. I <br />
begin by asking them if they know what table salt (sodium chloride) looks like and if they <br />
could point it out to me if they saw it. They <strong>of</strong> course claim they can, so I put up photos <strong>of</strong> <br />
sodium chloride and magnesium chloride and have them vote for which one is table salt. I <br />
then mention the difficulties associated with determining what atoms and molecules make up <br />
the Sun. These two examples seem to drive home the point that scientists need a clever way <br />
for determining what things are made <strong>of</strong>. <br />
<br />
Following the introduction, I have students answer a few pre‐questions about light (such as <br />
what it is and where it comes from) and I have them play with a diffraction grating and write <br />
down what they notice. We review their answers aloud so I can get a quick idea <strong>of</strong> how much <br />
material they remember from middle school. This leads into the PowerPoint presentation, <br />
where I discuss what light is and what the different colors represent. <br />
<br />
Once we review about light, the students work on the second part <strong>of</strong> the first handout, which <br />
includes writing electron configurations and filling out an electron configuration diagram. We <br />
review the answers aloud again, giving me a chance to gauge their current understanding. <br />
This is followed by a return to the PowerPoint presentation, where we quickly review filling <br />
out electron configuration diagrams. From here, the idea <strong>of</strong> absorption and emission are <br />
covered, and we discuss how each element gives <strong>of</strong>f certain colors when heated. <br />
<br />
This concludes the PowerPoint presentation, and leads directly into the flame test <br />
demonstration. I begin this activity by asking students for guesses <strong>of</strong> what color table salt <br />
(sodium chloride) will emit when lit on fire since it is a chemical they are familiar with and <br />
some might have seen it before. We then check the color. I continue to ask students what <br />
color they expect to be emitted, even though it is unlikely they will be able to immediately <br />
determine a trend – it is done more to keep students thinking, although I had no problem <br />
keeping the students engaged by the vibrant colors. Another thing to note is that it is best to <br />
save the salts SrCl2 (magenta), BaCl2 (green), and CuCl2 (blue) for last, as these emitted colors <br />
have the biggest impact with students. In order to make the colors more vibrant and to
ensure no contamination <strong>of</strong> sodium chloride, the flame test loop should be continually cleaned <br />
with HCl. <br />
<br />
Now that the students have seen and recorded the emitted colors, I hand out the second <br />
worksheet, which helps guide students through determining the periodic trends <strong>of</strong> color <strong>of</strong> <br />
light emitted. The trends are not very obvious and there are many subtleties involved, so I <br />
debrief the students afterwards by asking what results they saw and discussing why any <br />
breaks in the trends occurred. <br />
<br />
Procedure: <br />
• Teacher presents motivation for the upcoming activity (part <strong>of</strong> PowerPoint <br />
presentation) <br />
• Teacher provides students with a diffraction grating and first worksheet, which <br />
students will work on (Properties <strong>of</strong> Light: Pre‐Questions) for 5 minutes or so <br />
• Teacher and students review answers to pre‐questions <br />
• Teacher begins PowerPoint presentation on light and waves while students fill out <br />
blanks in notes (Properties <strong>of</strong> Light: Notes) <br />
• Once teacher finishes this part <strong>of</strong> the lesson, students work on second part <strong>of</strong> the first <br />
worksheet (Behavior <strong>of</strong> Electrons: Pre‐Questions) <br />
• Teacher and students review answers to pre‐questions <br />
• Teacher begins PowerPoint presentation on excitation <strong>of</strong> electrons while students fill <br />
out blanks in notes (Behavior <strong>of</strong> Electrons: Notes) <br />
• Teacher performs flame test demonstration, while students record colors emitted by <br />
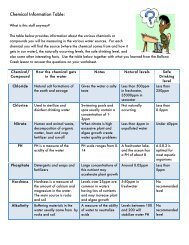
different chemicals on their worksheet (<strong>Flame</strong> <strong>Test</strong>: Data Table) <br />
o Have multiple chloride salts available <br />
o Add about 0.5 to 1.0 grams <strong>of</strong> each salt into its own empty beaker or flask <br />
o Add enough distilled water to each beaker/flask to make supersaturated salt <br />
solutions <br />
o Clean flame test loops with HCl before and after use (best if each salt sample has <br />
its own flame test loop) <br />
o Dip flame test loop in saturated salt solution, and place flame test loop in flame <br />
created by Bunsen burner <br />
o Repeat with each salt sample <br />
• Teacher hands out second worksheet and students complete the worksheet in class <br />
• Teacher and students review answers to worksheet and discuss advantages and <br />
disadvantages to flame testing <br />
• Teacher assigns the corresponding homework assignment for students to complete <br />
<br />
Materials List <br />
Students will need: <br />
• Diffraction gratings <br />
Teacher will need: <br />
• Safety goggles <br />
• Bunsen burner <br />
• <strong>Flame</strong> test loop <br />
• Various chloride salts and distilled water <br />
Safety Issues: <br />
This activity does require an open flame, so make sure to wear the proper safety equipment <br />
(goggles). The students do not need safety equipment as they are not participating in the <br />
demonstration, but they should be a safe distance away from the open flame at all times. <br />
<br />
<strong>Lesson</strong> Closure: <br />
Once the students have completed the second handout that helps them discover the periodic <br />
trends associated with flame testing, we discuss their results. Students will immediately <br />
notice breaks in the trends, so we discuss why this trend is not clear‐cut. If sodium <br />
contamination was prevalent in the demonstration, this is a good time to discuss why most <br />
compounds had a yellow/orange hue and how this can be a potential problem with flame <br />
testing. From here, I mention the various applications and tell students they now have the <br />
“opportunity” to investigate these applications further by completing the homework. <br />
<br />
The flame test is not a new idea, but the Powerpoint presentation, handouts, and homework <br />
are all original. <br />
<br />
Attachments: <br />
Handout 1 ‐ <strong>Flame</strong> <strong>Test</strong> Worksheet <br />
Handout 2 – <strong>Flame</strong> <strong>Test</strong> Thought Questions <br />
Powerpoint – <strong>Flame</strong> <strong>Test</strong> <strong>Activity</strong> <br />
<br />
List CA Science Standards addressed: <br />
• Atomic and Molecular Structure