Activity: What's the Story with Static Electricity? - UCLA
Activity: What's the Story with Static Electricity? - UCLA
Activity: What's the Story with Static Electricity? - UCLA
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Activity</strong>: What’s <strong>the</strong> <strong>Story</strong> <strong>with</strong> <strong>Static</strong> <strong>Electricity</strong>?<br />
Subject Area(s): Physical Science<br />
Lesson Title: What’s <strong>the</strong> story <strong>with</strong> static electricity?<br />
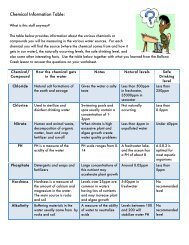
Large amount of static electricity built up on a person.<br />
http://www.funnyphotos.net.au/images/static-electricity-hair-stand-on-end1.jpg<br />
Grade Level: 8<br />
Time Required: 1 hour<br />
Summary<br />
In this activity/lesson students are introduced to <strong>the</strong> basis of static electricity. First <strong>the</strong>y are<br />
introduced to <strong>the</strong> structure of all matter, atoms. Specifically, <strong>the</strong> students learn that <strong>the</strong> structure<br />
of an atom is made up of sub-atomical particles (neutron, electron, proton) and relate <strong>the</strong> concept<br />
to explore static electricity, how it is created and its effects. Using a Van der Graaff generator<br />
for demonstration, students learn that static electricity refers to <strong>the</strong> build up of electric charges on<br />
<strong>the</strong> surface of objects. Students first make an observation (balloons on wall <strong>with</strong> static) and <strong>the</strong>n<br />
<strong>the</strong>y are asked to describe what <strong>the</strong>y think is happening. The goal of <strong>the</strong> activity is to guide <strong>the</strong><br />
students to make explorable questions about <strong>the</strong> concepts <strong>the</strong>y are learning that <strong>the</strong>y can test <strong>with</strong><br />
simple materials.<br />
Keywords<br />
Atoms, static electricity, Van der Graaf<br />
Educational Standards
CA Science Standard:<br />
Grade 4 1E: Students know electrically charged objects attract or repel each o<strong>the</strong>r.<br />
Grade 8: 3a. Students know <strong>the</strong> structure of <strong>the</strong> atom and know it is composed of protons,<br />
neutrons, and electrons.<br />
3b. Students know that compounds are formed by combining two or more different elements and<br />
that compounds have properties that are different from <strong>the</strong>ir constituent elements.<br />
Material List:<br />
Van der Graaff generator (<strong>the</strong> generator comes <strong>with</strong> <strong>the</strong> supplies)<br />
(can you add <strong>the</strong> catalog number and purchasing detail, I couldn’t find it on line)<br />
Metallic balls<br />
Puffed wheat<br />
Styrofoam cup and balls<br />
Pre-Requisite Knowledge<br />
Students know electrically charged objects attract or repel each o<strong>the</strong>r.<br />
Learning Objectives<br />
After this lesson, students should be able to:<br />
Describe <strong>the</strong> structure of an atom.<br />
Define sub-atomical particles<br />
Understand that things are composed of more than one element <strong>with</strong> unique properties.<br />
Describe how static electricity forms.<br />
Introduction / Motivation<br />
All matter is made up of atoms. Atoms (Figure 3) are <strong>the</strong> basic building blocks of all matter.<br />
They have a nucleus that consists of <strong>the</strong> sub-atomical particles, protons, neutrons and electrons.<br />
To draw in <strong>the</strong> students describe <strong>the</strong> atomic make up of a human body. How many atoms are<br />
you made of? Proceed by having <strong>the</strong> students take a guess at <strong>the</strong> number of atoms that make up<br />
a human body (average weight of 70kg).<br />
~99% of human body is made up of:<br />
Hydrogen<br />
Oxygen<br />
Carbon<br />
Nitrogen<br />
Calcium<br />
Phosphorus
1% is made up of trace elements<br />
For simplicity concentrate on <strong>the</strong> most abundant: H, O, C:<br />
(write <strong>the</strong> guesses on <strong>the</strong> board)<br />
Then on <strong>the</strong> board write down <strong>the</strong> amount of atoms for each element and add <strong>the</strong>m up:<br />
H: 4.7*1027<br />
O: 1.8*1027<br />
C: 7.0*1026<br />
Total: 7*1027<br />
(You can give <strong>the</strong> closest prediction a prize)<br />
Structure of an atom.<br />
http://www.sciencemadesimple.com/static.html<br />
Procedure<br />
Have <strong>the</strong> students rub a balloon on <strong>the</strong>ir heads and attempt to put it on <strong>the</strong> wall. Ask <strong>the</strong>m what<br />
<strong>the</strong>y think is happening? Next move on to use <strong>the</strong> Van der Graaff generator (Figure 2 shows <strong>the</strong><br />
separation of charges) and explain <strong>the</strong> parts and have <strong>the</strong> students predict <strong>the</strong> demo.<br />
Allow <strong>the</strong> students to do <strong>the</strong>ir own testing using <strong>the</strong> supplies that come <strong>with</strong> <strong>the</strong> generator. Have<br />
<strong>the</strong> students make a team and give <strong>the</strong>m one of <strong>the</strong> supplies and predict what <strong>the</strong> effect will be.<br />
The students must come up <strong>with</strong> <strong>the</strong> prediction and explanation <strong>the</strong>n <strong>the</strong>y can test it in front of<br />
<strong>the</strong> class.
Van der Graaff Generator.<br />
http://wpcontent.answers.com/wikipedia/commons/thumb/c/c2/Van_de_graaf_generator.svg/320<br />
px-Van_de_graaf_generator.svg.png<br />
Lesson Background & Concepts for Teachers<br />
Additional information:<br />
The following background information was obtained from:<br />
http://www.sciencemadesimple.com/static.html<br />
You walk across <strong>the</strong> rug, reach for <strong>the</strong> doorknob and..........ZAP!!! You get a static shock. Or,<br />
you come inside from <strong>the</strong> cold, pull off your hat and......BOING!!! <strong>Static</strong> hair - static electricity<br />
makes your hair stand straight out from your head. What is going on here? And why is static<br />
more of a problem in <strong>the</strong> winter?<br />
To understand static electricity, we have to learn a little bit about <strong>the</strong> nature of matter. Or in o<strong>the</strong>r<br />
words, what is all <strong>the</strong> stuff around us made of? Atoms.<br />
Everything we see is made up of tiny little parts called atoms. The atoms are made of even<br />
smaller parts. These are called protons, electrons and neutrons. They are very different from each<br />
o<strong>the</strong>r in many ways. One way <strong>the</strong>y are different is <strong>the</strong>ir "charge." Protons have a positive (+)<br />
charge. Electrons have a negative (-) charge. Neutrons have no charge.<br />
Usually, atoms have <strong>the</strong> same number of electrons and protons. Then <strong>the</strong> atom has no charge, it<br />
is "neutral." But if you rub things toge<strong>the</strong>r, electrons can move from one atom to ano<strong>the</strong>r. Some<br />
atoms get extra electrons. They have a negative charge. O<strong>the</strong>r atoms lose electrons. They have a<br />
positive charge. When charges are separated like this, it is called static electricity.<br />
If two things have different charges, <strong>the</strong>y attract, or pull towards each o<strong>the</strong>r. If two things have<br />
<strong>the</strong> same charge, <strong>the</strong>y repel, or push away from each o<strong>the</strong>r.
So, why does your hair stand up after you take your hat off? When you pull your hat off, it rubs<br />
against your hair. Electrons move from your hair to <strong>the</strong> hat. Now each of <strong>the</strong> hairs has <strong>the</strong> same<br />
positive charge. Things <strong>with</strong> <strong>the</strong> same charge repel each o<strong>the</strong>r. So <strong>the</strong> hairs try to move away<br />
from each o<strong>the</strong>r. The far<strong>the</strong>st <strong>the</strong>y can get is to stand up and away from all <strong>the</strong> o<strong>the</strong>r hairs.<br />
If you walk across a carpet, electrons move from <strong>the</strong> rug to you. Now you have extra electrons.<br />
Touch a door knob and ZAP! The electrons move from you to <strong>the</strong> knob. You get a shock.<br />
Copyright © 1995-2009, Science Made Simple, Inc. All Rights Reserved.<br />
PO Box 503, Voorhees, NJ 08043<br />
Vocabulary / Definitions<br />
Word Definition<br />
Atoms Building block of all matter<br />
<strong>Electricity</strong> The movement of electrical charge<br />
Electrons Subatomical particle made up of a negative charge rotates around nucleus of<br />
an atom<br />
Nucleus The nucleus of an atom consists of protons, neutrons and electrons<br />
Neutrons Subatomical particle <strong>with</strong> neutral charge<br />
Protons Subatomical particle <strong>with</strong> positive charge<br />
Van der Graaff<br />
generator<br />
Lesson Closure<br />
Electrostatic generator that uses a belt and metal spheres to separate charges<br />
and cause a static electrical effect<br />
End <strong>the</strong> activity by making a human chain and transferring <strong>the</strong> electricity from person to person.<br />
Have <strong>the</strong> students predict how <strong>the</strong> experiment would work best. For example <strong>with</strong> more people<br />
less people, what types of objects or substances would help transfer <strong>the</strong> shock easier (water,<br />
plastic, ect.).<br />
References<br />
http://www.sciencemadesimple.com/static.html<br />
http://www.howstuffworks.com/vdg.htm<br />
http://education.jlab.org/qa/mathatom_04.html
Owner<br />
Gloria C. Preza and Robin Nagle<br />
Contributors<br />
<strong>UCLA</strong> GK-12 workshop instructed by Warren Essey<br />
Brian Gabrich