Activity: What's the Story with Static Electricity? - UCLA
Activity: What's the Story with Static Electricity? - UCLA
Activity: What's the Story with Static Electricity? - UCLA
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
CA Science Standard:<br />
Grade 4 1E: Students know electrically charged objects attract or repel each o<strong>the</strong>r.<br />
Grade 8: 3a. Students know <strong>the</strong> structure of <strong>the</strong> atom and know it is composed of protons,<br />
neutrons, and electrons.<br />
3b. Students know that compounds are formed by combining two or more different elements and<br />
that compounds have properties that are different from <strong>the</strong>ir constituent elements.<br />
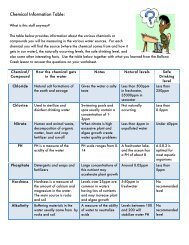
Material List:<br />
Van der Graaff generator (<strong>the</strong> generator comes <strong>with</strong> <strong>the</strong> supplies)<br />
(can you add <strong>the</strong> catalog number and purchasing detail, I couldn’t find it on line)<br />
Metallic balls<br />
Puffed wheat<br />
Styrofoam cup and balls<br />
Pre-Requisite Knowledge<br />
Students know electrically charged objects attract or repel each o<strong>the</strong>r.<br />
Learning Objectives<br />
After this lesson, students should be able to:<br />
Describe <strong>the</strong> structure of an atom.<br />
Define sub-atomical particles<br />
Understand that things are composed of more than one element <strong>with</strong> unique properties.<br />
Describe how static electricity forms.<br />
Introduction / Motivation<br />
All matter is made up of atoms. Atoms (Figure 3) are <strong>the</strong> basic building blocks of all matter.<br />
They have a nucleus that consists of <strong>the</strong> sub-atomical particles, protons, neutrons and electrons.<br />
To draw in <strong>the</strong> students describe <strong>the</strong> atomic make up of a human body. How many atoms are<br />
you made of? Proceed by having <strong>the</strong> students take a guess at <strong>the</strong> number of atoms that make up<br />
a human body (average weight of 70kg).<br />
~99% of human body is made up of:<br />
Hydrogen<br />
Oxygen<br />
Carbon<br />
Nitrogen<br />
Calcium<br />
Phosphorus