Brochure for Business Partners - Kyocera
Brochure for Business Partners - Kyocera
Brochure for Business Partners - Kyocera
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
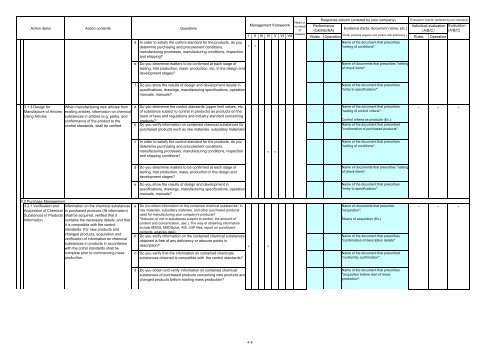
Action items Action contents Questions<br />
3.1.3 Design <strong>for</strong><br />
Manufacture of Articles<br />
Using Articles<br />
When manufacturing new articles from<br />
existing articles, in<strong>for</strong>mation on chemical<br />
substances in articles (e.g. parts), and<br />
con<strong>for</strong>mance of the product to the<br />
control standards, shall be verified.<br />
3.2 Purchase Management<br />
3.2.1 Verification and In<strong>for</strong>mation on the chemical substances<br />
Acquisition of Chemical in purchased products (IN in<strong>for</strong>mation)<br />
Substances in Products shall be acquired, verified that it<br />
In<strong>for</strong>mation<br />
contains the necessary details, and that<br />
it is compatible with the control<br />
standards. For new products and<br />
changed products, acquisition and<br />
verification of in<strong>for</strong>mation on chemical<br />
substances in products in accordance<br />
with the cotrol standards shall be<br />
complete prior to commencing mass<br />
production.<br />
d In order to satisfy the control standard <strong>for</strong> the products, do you<br />
determine purchasing and procurement conditions,<br />
manufacturing processes, manufacturing conditions, inspection<br />
and shipping?<br />
e Do you determine matters to be confirmed at each stage of<br />
testing, trial production, mass- production, etc. in the design and<br />
development stages?<br />
f Do you show the results of design and development results in<br />
specifications, drawings, manufacturing specifications, operation<br />
manuals, manuals?<br />
a Do you determine the control standards (upper limit values, etc.<br />
of substance subject to control in products) as products on the<br />
basis of laws and regulations and industry standard concerning<br />
products?<br />
b Do you verify in<strong>for</strong>mation on contained chemical substances <strong>for</strong><br />
purchased products such as raw materials, subsidiary materials?<br />
c In order to satisfy the control standard <strong>for</strong> the products, do you<br />
determine purchasing and procurement conditions,<br />
manufacturing processes, manufacturing conditions, inspection<br />
and shipping conditions?<br />
d Do you determine matters to be confirmed at each stage of<br />
testing, trial production, mass- production in the design and<br />
development stages?<br />
e Do you show the results of design and development in<br />
specifications, drawings, manufacturing specifications, operation<br />
manuals, manuals?<br />
a Do you obtain in<strong>for</strong>mation on the contained chemical substances* in<br />
raw materials, subsidiary materials, and other purchased products<br />
used <strong>for</strong> manufacturing your company's products?<br />
*Inclusion or not in substances subject to control, the amount of<br />
content and concentration, use ( The way of obtaining in<strong>for</strong>mation<br />
include MSDS, MSDSplus, AIS, JGP files, report on constituent<br />
contents, analysis data).<br />
b Do you verify in<strong>for</strong>mation on the contained chemical substances<br />
obtained is free of any deficiency or obscure points in<br />
description?<br />
c Do you verify that the in<strong>for</strong>mation on contained chemicals<br />
substances obtained is compatible with the control standards?<br />
d Do you obtain and verify in<strong>for</strong>mation on contained chemical<br />
substances of purchased products concerning new products and<br />
changed products be<strong>for</strong>e starting mass production?<br />
Management framework<br />
Need or<br />
no-need<br />
of<br />
Response column (entered by your company)<br />
Per<strong>for</strong>mance<br />
Evidence (facts, document name, etc.)<br />
(OK/NG/NA)<br />
Evaluation column (entered by our company)<br />
Individual evaluation Evaluation<br />
(A/B/C) (A/B/C)<br />
I II III IV V VI VII<br />
answer<br />
Rules<br />
(Enter practical aspects and portion with deficiency.)<br />
Operation<br />
Name of the document that prescribes<br />
Rules Operation<br />
v v<br />
"setting of conditions":<br />
v<br />
4/8<br />
v v<br />
v<br />
Name of documents that prescribes "setting<br />
of check items":<br />
Name of the document that prescribes<br />
"entry to specifications":<br />
Name of the document that prescribes<br />
"setting of control criteria":<br />
Control criteria as products (Ex.):<br />
Name of the document that prescribed<br />
"confirmation of purchased products":<br />
Name of the document that prescribes<br />
"setting of conditions":<br />
Name of documents that prescribes "setting<br />
of check items":<br />
Name of the document that prescribes<br />
"entry to specifications":<br />
Name of documents that prescribe<br />
"acquisition":<br />
Means of acquisition (Ex.):<br />
Name of the document that prescribes<br />
"confirmation of description details":<br />
Name of the document that prescribed<br />
"con<strong>for</strong>mity confirmation":<br />
Name of the document that prescribes<br />
"acquisition be<strong>for</strong>e start of mass<br />
production":<br />
- - -<br />
-<br />
- -