Structural, Spectral, Biological and Electrochemical Studies Of Some ...
Structural, Spectral, Biological and Electrochemical Studies Of Some ...
Structural, Spectral, Biological and Electrochemical Studies Of Some ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
an additional 22 minutes. The crystals were collected by filtration, washed well with<br />
water <strong>and</strong> dried under a heat lamp. The crude product was recrystallised from a<br />
mixture of 50 mL of ethanol <strong>and</strong> 25 mL of water. This gave 10.8 g (81%) of stout<br />
colourless rods of 4-methyl-4-phenyl-3-thiosemicarbazide, mp 124°C.<br />
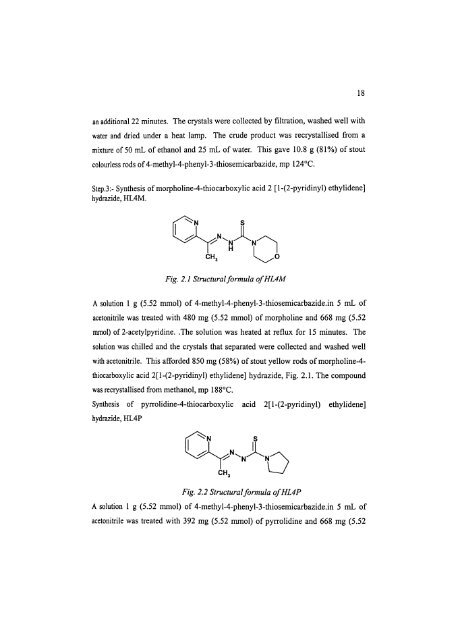
Step.3:- Synthesis of morpholine-4-thiocarboxylic acid 2 [1-(2-pyridinyl) ethylidene]<br />
hydrazide, HL4M.<br />
Fig. 2.1 <strong>Structural</strong> formula ofHL4M<br />
A solution 1 g (5.52 mmol) of 4-methyl-4-phenyl-3-thiosemicarbazide.in 5 mL of<br />
acetonitrile was treated with 480 mg (5.52 mmol) of morpholine <strong>and</strong> 668 mg (5.52<br />
mmol) of 2-acetylpyridine..The solution was heated at reflux for 15 minutes. The<br />
solution was chilled <strong>and</strong> the crystals that separated were collected <strong>and</strong> washed well<br />
with acetonitrile. This afforded 850 mg (58%) ofstout yellow rods ofmorpholine-4<br />
thiocarboxylic acid 2[1-(2-pyridinyl) ethylidene] hydrazide, Fig. 2.1. The compound<br />
was recrystallised from methanol, mp 188°C.<br />
Synthesis of pyrrolidine-4-thiocarboxylic acid 2[1-(2-pyridinyl) ethylidene]<br />
hydrazide, HL4P<br />
Fig. 2.2 <strong>Structural</strong>formula ofHL4P<br />
A solution 1 g (5.52 mmol) of 4-methyl-4-phenyl-3-thiosemicarbazide.in 5 mL of<br />
acetonitrile was treated with 392 mg (5.52 mmol) of pyrrolidine <strong>and</strong> 668 mg (5.52<br />
18