Evaluation of bactericidal and fungicidal activity of ferrocenyl or phenyl derivatives...
Ferrocene compounds
Ferrocene compounds
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
complexes have been synthesized. Some compounds demonstrated<br />
excellent cytotoxic <strong>activity</strong> on both h<strong>or</strong>mone-dependent <strong>and</strong><br />
h<strong>or</strong>mone-independent cancer cells. F<strong>or</strong> example, compounds 1<br />
(hydroxyferrocifen) [12], 2 (ferrociphenol) [12], 3 [14] <strong>and</strong> 4 [14] are<br />
very active on h<strong>or</strong>mone-independent MDA-MB-231 cancer cells,<br />
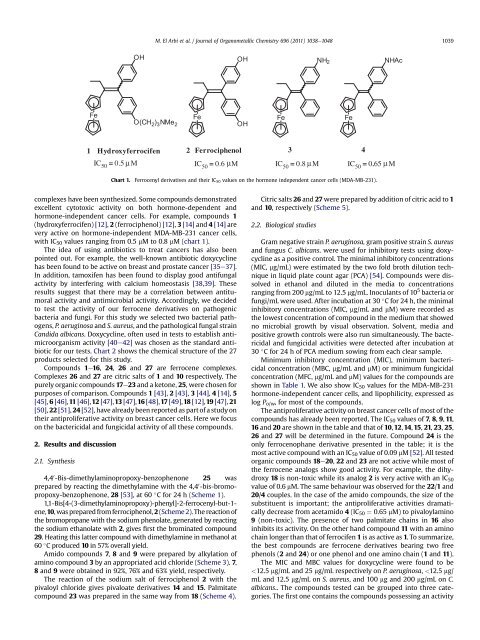
with IC50 values ranging from 0.5 mM to 0.8 mM (chart 1).<br />
The idea <strong>of</strong> using antibiotics to treat cancers has also been<br />
pointed out. F<strong>or</strong> example, the well-known antibiotic doxycycline<br />
has been found to be active on breast <strong>and</strong> prostate cancer [35e37].<br />
In addition, tamoxifen has been found to display good antifungal<br />
<strong>activity</strong> by interfering with calcium homeostasis [38,39]. These<br />
results suggest that there may be a c<strong>or</strong>relation between antitum<strong>or</strong>al<br />
<strong>activity</strong> <strong>and</strong> antimicrobial <strong>activity</strong>. Acc<strong>or</strong>dingly, we decided<br />
to test the <strong>activity</strong> <strong>of</strong> our ferrocene <strong>derivatives</strong> on pathogenic<br />
bacteria <strong>and</strong> fungi. F<strong>or</strong> this study we selected two bacterial pathogens,<br />
P. aeruginosa <strong>and</strong> S. aureus, <strong>and</strong> the pathological fungal strain<br />
C<strong>and</strong>ida albicans. Doxycycline, <strong>of</strong>ten used in tests to establish antimicro<strong>or</strong>ganism<br />
<strong>activity</strong> [40e42] was chosen as the st<strong>and</strong>ard antibiotic<br />
f<strong>or</strong> our tests. Chart 2 shows the chemical structure <strong>of</strong> the 27<br />
products selected f<strong>or</strong> this study.<br />
Compounds 1e16, 24, 26 <strong>and</strong> 27 are ferrocene complexes.<br />
Complexes 26 <strong>and</strong> 27 are citric salts <strong>of</strong> 1 <strong>and</strong> 10 respectively. The<br />
purely <strong>or</strong>ganic compounds 17e23 <strong>and</strong> a ketone, 25, were chosen f<strong>or</strong><br />
purposes <strong>of</strong> comparison. Compounds 1 [43], 2 [43], 3 [44], 4 [14], 5<br />
[45], 6 [46],11 [46],12 [47],13 [47],16 [48],17 [49],18 [12],19 [47], 21<br />
[50], 22 [51], 24 [52], have already been rep<strong>or</strong>ted as part <strong>of</strong> a study on<br />
their antiproliferative <strong>activity</strong> on breast cancer cells. Here we focus<br />
on the <strong>bactericidal</strong> <strong>and</strong> <strong>fungicidal</strong> <strong>activity</strong> <strong>of</strong> all these compounds.<br />
2. Results <strong>and</strong> discussion<br />
2.1. Synthesis<br />
Fe<br />
OH<br />
O(CH 2 ) 3 NMe 2<br />
1 Hydroxyferrocifen 2 Ferrociphenol<br />
3 4<br />
IC50 = 0.5 µ M<br />
IC50 = 0.6 µ M IC50 = 0.8 µ M IC50 = 0.65 . µ M<br />
4,4 0 -Bis-dimethylaminopropoxy-benzophenone 25 was<br />
prepared by reacting the dimethylamine with the 4,4 0 -bis-bromopropoxy-benzophenone,<br />
28 [53], at60 C f<strong>or</strong> 24 h (Scheme 1).<br />
1,1-Bis[4-(3-dimethylaminopropoxy)-<strong>phenyl</strong>]-2-<strong>ferrocenyl</strong>-but-1ene,10,<br />
was prepared from ferrociphenol, 2 (Scheme 2). The reaction <strong>of</strong><br />
the bromopropane with the sodium phenolate, generated by reacting<br />
the sodium ethanolate with 2, givesfirst the brominated compound<br />
29. Heating this latter compound with dimethylamine in methanol at<br />
60 C produced 10 in 57% overall yield.<br />
Amido compounds 7, 8 <strong>and</strong> 9 were prepared by alkylation <strong>of</strong><br />
amino compound 3 by an appropriated acid chl<strong>or</strong>ide (Scheme 3). 7,<br />
8 <strong>and</strong> 9 were obtained in 92%, 76% <strong>and</strong> 63% yield, respectively.<br />
The reaction <strong>of</strong> the sodium salt <strong>of</strong> ferrociphenol 2 with the<br />
pivaloyl chl<strong>or</strong>ide gives pivaloate <strong>derivatives</strong> 14 <strong>and</strong> 15. Palmitate<br />
compound 23 was prepared in the same way from 18 (Scheme 4).<br />
M. El Arbi et al. / Journal <strong>of</strong> Organometallic Chemistry 696 (2011) 1038e1048 1039<br />
Fe<br />
OH<br />
OH<br />
Chart 1. Ferrocenyl <strong>derivatives</strong> <strong>and</strong> their IC50 values on the h<strong>or</strong>mone independent cancer cells (MDA-MB-231).<br />
Fe<br />
Citric salts 26 <strong>and</strong> 27 were prepared by addition <strong>of</strong> citric acid to 1<br />
<strong>and</strong> 10, respectively (Scheme 5).<br />
2.2. Biological studies<br />
NH2<br />
Fe<br />
NHAc<br />
Gram negative strain P. aeruginosa, gram positive strain S. aureus<br />
<strong>and</strong> fungus C. albicans. were used f<strong>or</strong> inhibit<strong>or</strong>y tests using doxycycline<br />
as a positive control. The minimal inhibit<strong>or</strong>y concentrations<br />
(MIC, mg/mL) were estimated by the two fold broth dilution technique<br />
in liquid plate count agar (PCA) [54]. Compounds were dissolved<br />
in ethanol <strong>and</strong> diluted in the media to concentrations<br />
ranging from 200 mg/mL to 12.5 mg/mL. Inoculants <strong>of</strong> 10 5 bacteria <strong>or</strong><br />
fungi/mL were used. After incubation at 30 C f<strong>or</strong> 24 h, the minimal<br />
inhibit<strong>or</strong>y concentrations (MIC, mg/mL <strong>and</strong> mM) were rec<strong>or</strong>ded as<br />
the lowest concentration <strong>of</strong> compound in the medium that showed<br />
no microbial growth by visual observation. Solvent, media <strong>and</strong><br />
positive growth controls were also run simultaneously. The <strong>bactericidal</strong><br />
<strong>and</strong> <strong>fungicidal</strong> activities were detected after incubation at<br />
30 C f<strong>or</strong> 24 h <strong>of</strong> PCA medium sowing from each clear sample.<br />
Minimum inhibit<strong>or</strong>y concentration (MIC), minimum <strong>bactericidal</strong><br />
concentration (MBC, mg/mL <strong>and</strong> mM) <strong>or</strong> minimum <strong>fungicidal</strong><br />
concentration (MFC, mg/mL <strong>and</strong> mM) values f<strong>or</strong> the compounds are<br />
shown in Table 1. We also show IC50 values f<strong>or</strong> the MDA-MB-231<br />
h<strong>or</strong>mone-independent cancer cells, <strong>and</strong> lipophilicity, expressed as<br />
log Po/w, f<strong>or</strong> most <strong>of</strong> the compounds.<br />
The antiproliferative <strong>activity</strong> on breast cancer cells <strong>of</strong> most <strong>of</strong> the<br />
compounds has already been rep<strong>or</strong>ted. The IC50 values <strong>of</strong> 7, 8, 9, 11,<br />
16 <strong>and</strong> 20 are shown in the table <strong>and</strong> that <strong>of</strong> 10, 12, 14, 15, 21, 23, 25,<br />
26 <strong>and</strong> 27 will be determined in the future. Compound 24 is the<br />
only ferrocenophane derivative presented in the table; it is the<br />
most active compound with an IC50 value <strong>of</strong> 0.09 mM [52]. All tested<br />
<strong>or</strong>ganic compounds 18e20, 22 <strong>and</strong> 23 are not active while most <strong>of</strong><br />
the ferrocene analogs show good <strong>activity</strong>. F<strong>or</strong> example, the dihydroxy<br />
18 is non-toxic while its analog 2 is very active with an IC50<br />
value <strong>of</strong> 0.6 mM. The same behaviour was observed f<strong>or</strong> the 22/1 <strong>and</strong><br />
20/4 couples. In the case <strong>of</strong> the amido compounds, the size <strong>of</strong> the<br />
substituent is imp<strong>or</strong>tant; the antiproliferative activities dramatically<br />
decrease from acetamido 4 (IC50 ¼ 0.65 mM) to pivaloylamino<br />
9 (non-toxic). The presence <strong>of</strong> two palmitate chains in 16 also<br />
inhibits its <strong>activity</strong>. On the other h<strong>and</strong> compound 11 with an amino<br />
chain longer than that <strong>of</strong> ferrocifen 1 is as active as 1. To summarize,<br />
the best compounds are ferrocene <strong>derivatives</strong> bearing two free<br />
phenols (2 <strong>and</strong> 24) <strong>or</strong> one phenol <strong>and</strong> one amino chain (1 <strong>and</strong> 11).<br />
The MIC <strong>and</strong> MBC values f<strong>or</strong> doxycycline were found to be<br />