Phase transition and density of subducted MORB crust in the lower ...
Phase transition and density of subducted MORB crust in the lower ...
Phase transition and density of subducted MORB crust in the lower ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
248<br />
K. Hirose et al. / Earth <strong>and</strong> Planetary Science Letters 237 (2005) 239–251<br />
<strong>of</strong> CaFe 2O 4-type Al-phase is larger <strong>the</strong> PREM <strong>density</strong><br />
even at high temperatures (Fig. 5d). It is noted, however,<br />
that chemical composition <strong>of</strong> CaFe 2O 4-type Alphase<br />
is variable depend<strong>in</strong>g on <strong>the</strong> bulk <strong>MORB</strong> composition<br />
as summarized by Guignot <strong>and</strong> Andrault<br />
[36], which significantly affects <strong>the</strong> m<strong>in</strong>eral <strong>density</strong>.<br />
Ono et al. [7] calculated remarkably <strong>lower</strong> densities<br />
for this m<strong>in</strong>eral primarily due to <strong>the</strong> difference <strong>in</strong><br />
chemical composition.<br />
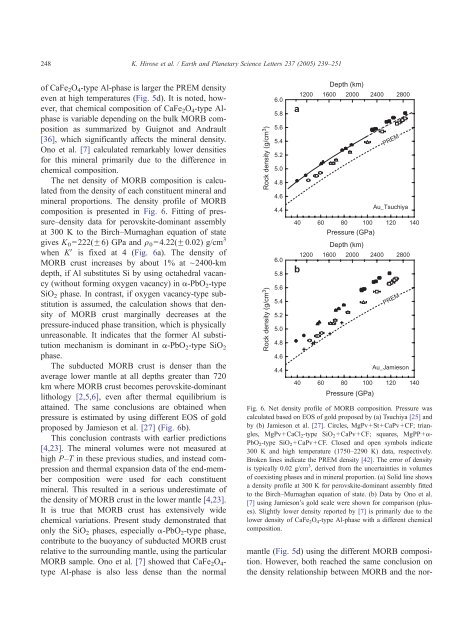
The net <strong>density</strong> <strong>of</strong> <strong>MORB</strong> composition is calculated<br />
from <strong>the</strong> <strong>density</strong> <strong>of</strong> each constituent m<strong>in</strong>eral <strong>and</strong><br />
m<strong>in</strong>eral proportions. The <strong>density</strong> pr<strong>of</strong>ile <strong>of</strong> <strong>MORB</strong><br />
composition is presented <strong>in</strong> Fig. 6. Fitt<strong>in</strong>g <strong>of</strong> pressure–<strong>density</strong><br />
data for perovskite-dom<strong>in</strong>ant assembly<br />
at 300 K to <strong>the</strong> Birch–Murnaghan equation <strong>of</strong> state<br />
gives K0=222(F6) GPa <strong>and</strong> q0=4.22(F0.02) g/cm 3<br />
when KV is fixed at 4 (Fig. 6a). The <strong>density</strong> <strong>of</strong><br />
<strong>MORB</strong> <strong>crust</strong> <strong>in</strong>creases by about 1% at ~2400-km<br />
depth, if Al substitutes Si by us<strong>in</strong>g octahedral vacancy<br />
(without form<strong>in</strong>g oxygen vacancy) <strong>in</strong> a-PbO2-type<br />
SiO2 phase. In contrast, if oxygen vacancy-type substitution<br />
is assumed, <strong>the</strong> calculation shows that <strong>density</strong><br />
<strong>of</strong> <strong>MORB</strong> <strong>crust</strong> marg<strong>in</strong>ally decreases at <strong>the</strong><br />
pressure-<strong>in</strong>duced phase <strong>transition</strong>, which is physically<br />
unreasonable. It <strong>in</strong>dicates that <strong>the</strong> former Al substitution<br />
mechanism is dom<strong>in</strong>ant <strong>in</strong> a-PbO 2-type SiO 2<br />
phase.<br />
The <strong>subducted</strong> <strong>MORB</strong> <strong>crust</strong> is denser than <strong>the</strong><br />
average <strong>lower</strong> mantle at all depths greater than 720<br />
km where <strong>MORB</strong> <strong>crust</strong> becomes perovskite-dom<strong>in</strong>ant<br />
lithology [2,5,6], even after <strong>the</strong>rmal equilibrium is<br />
atta<strong>in</strong>ed. The same conclusions are obta<strong>in</strong>ed when<br />
pressure is estimated by us<strong>in</strong>g different EOS <strong>of</strong> gold<br />
proposed by Jamieson et al. [27] (Fig. 6b).<br />
This conclusion contrasts with earlier predictions<br />
[4,23]. The m<strong>in</strong>eral volumes were not measured at<br />
high P–T <strong>in</strong> <strong>the</strong>se previous studies, <strong>and</strong> <strong>in</strong>stead compression<br />
<strong>and</strong> <strong>the</strong>rmal expansion data <strong>of</strong> <strong>the</strong> end-member<br />
composition were used for each constituent<br />
m<strong>in</strong>eral. This resulted <strong>in</strong> a serious underestimate <strong>of</strong><br />
<strong>the</strong> <strong>density</strong> <strong>of</strong> <strong>MORB</strong> <strong>crust</strong> <strong>in</strong> <strong>the</strong> <strong>lower</strong> mantle [4,23].<br />
It is true that <strong>MORB</strong> <strong>crust</strong> has extensively wide<br />
chemical variations. Present study demonstrated that<br />
only <strong>the</strong> SiO2 phases, especially a-PbO2-type phase,<br />
contribute to <strong>the</strong> buoyancy <strong>of</strong> <strong>subducted</strong> <strong>MORB</strong> <strong>crust</strong><br />
relative to <strong>the</strong> surround<strong>in</strong>g mantle, us<strong>in</strong>g <strong>the</strong> particular<br />
<strong>MORB</strong> sample. Ono et al. [7] showed that CaFe2O4type<br />
Al-phase is also less dense than <strong>the</strong> normal<br />
Rock <strong>density</strong> (g/cm 3 ) Rock <strong>density</strong> (g/cm 3 )<br />
6.0<br />
5.8<br />
5.6<br />
5.4<br />
5.2<br />
5.0<br />
4.8<br />
4.6<br />
4.4<br />
6.0<br />
5.8<br />
5.6<br />
5.4<br />
5.2<br />
5.0<br />
4.8<br />
4.6<br />
4.4<br />
a<br />
40 60 80 100 120 140<br />
b<br />
Depth (km)<br />
1200 1600 2000 2400 2800<br />
Pressure (GPa)<br />
Depth (km)<br />
1200 1600 2000 2400 2800<br />
40 60 80 100 120 140<br />
Pressure (GPa)<br />
PREM<br />
Au_Tsuchiya<br />
PREM<br />
Au_Jamieson<br />
Fig. 6. Net <strong>density</strong> pr<strong>of</strong>ile <strong>of</strong> <strong>MORB</strong> composition. Pressure was<br />
calculated based on EOS <strong>of</strong> gold proposed by (a) Tsuchiya [25] <strong>and</strong><br />
by (b) Jamieson et al. [27]. Circles, MgPv+St+CaPv+CF; triangles,<br />
MgPv+CaCl 2-type SiO 2+CaPv+CF; squares, MgPP+a-<br />
PbO2-type SiO2+CaPv+CF. Closed <strong>and</strong> open symbols <strong>in</strong>dicate<br />
300 K <strong>and</strong> high temperature (1750–2290 K) data, respectively.<br />
Broken l<strong>in</strong>es <strong>in</strong>dicate <strong>the</strong> PREM <strong>density</strong> [42]. The error <strong>of</strong> <strong>density</strong><br />
is typically 0.02 g/cm 3 , derived from <strong>the</strong> uncerta<strong>in</strong>ties <strong>in</strong> volumes<br />
<strong>of</strong> coexist<strong>in</strong>g phases <strong>and</strong> <strong>in</strong> m<strong>in</strong>eral proportion. (a) Solid l<strong>in</strong>e shows<br />
a <strong>density</strong> pr<strong>of</strong>ile at 300 K for perovskite-dom<strong>in</strong>ant assembly fitted<br />
to <strong>the</strong> Birch–Murnaghan equation <strong>of</strong> state. (b) Data by Ono et al.<br />
[7] us<strong>in</strong>g Jamieson’s gold scale were shown for comparison (pluses).<br />
Slightly <strong>lower</strong> <strong>density</strong> reported by [7] is primarily due to <strong>the</strong><br />
<strong>lower</strong> <strong>density</strong> <strong>of</strong> CaFe 2O 4-type Al-phase with a different chemical<br />
composition.<br />
mantle (Fig. 5d) us<strong>in</strong>g <strong>the</strong> different <strong>MORB</strong> composition.<br />
However, both reached <strong>the</strong> same conclusion on<br />
<strong>the</strong> <strong>density</strong> relationship between <strong>MORB</strong> <strong>and</strong> <strong>the</strong> nor-