Phase transition and density of subducted MORB crust in the lower ...
Phase transition and density of subducted MORB crust in the lower ...
Phase transition and density of subducted MORB crust in the lower ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Density (g/cm 3 )<br />
Density (g/cm 3 )<br />
Density (g/cm 3 )<br />
Density (g/cm 3 )<br />
6.5<br />
6.0<br />
5.5<br />
5.0<br />
4.5<br />
5.6<br />
5.4<br />
5.2<br />
5.0<br />
4.8<br />
4.6<br />
5.6<br />
5.4<br />
5.2<br />
5.0<br />
4.8<br />
4.6<br />
5.6<br />
5.4<br />
5.2<br />
5.0<br />
4.8<br />
4.6<br />
4.4<br />
a<br />
b<br />
c<br />
d<br />
K. Hirose et al. / Earth <strong>and</strong> Planetary Science Letters 237 (2005) 239–251 247<br />
PREM<br />
MgPv+MgPP<br />
SiO 2 -phases<br />
CaPv<br />
CF<br />
40 60 80 100 120 140<br />
Pressure (GPa)<br />
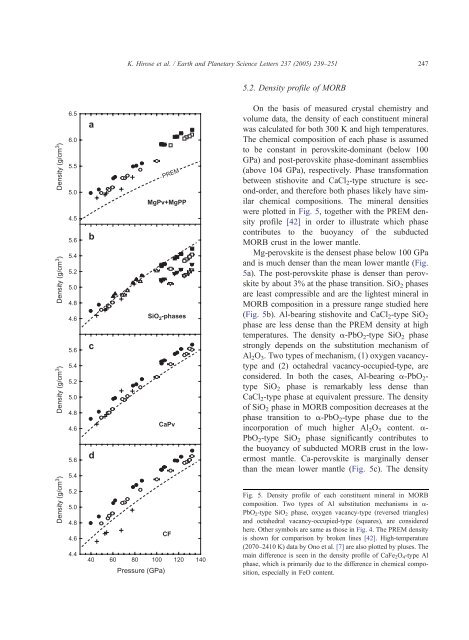
5.2. Density pr<strong>of</strong>ile <strong>of</strong> <strong>MORB</strong><br />
On <strong>the</strong> basis <strong>of</strong> measured crystal chemistry <strong>and</strong><br />
volume data, <strong>the</strong> <strong>density</strong> <strong>of</strong> each constituent m<strong>in</strong>eral<br />
was calculated for both 300 K <strong>and</strong> high temperatures.<br />
The chemical composition <strong>of</strong> each phase is assumed<br />
to be constant <strong>in</strong> perovskite-dom<strong>in</strong>ant (below 100<br />
GPa) <strong>and</strong> post-perovskite phase-dom<strong>in</strong>ant assemblies<br />
(above 104 GPa), respectively. <strong>Phase</strong> transformation<br />
between stishovite <strong>and</strong> CaCl 2-type structure is second-order,<br />
<strong>and</strong> <strong>the</strong>refore both phases likely have similar<br />
chemical compositions. The m<strong>in</strong>eral densities<br />
were plotted <strong>in</strong> Fig. 5, toge<strong>the</strong>r with <strong>the</strong> PREM <strong>density</strong><br />
pr<strong>of</strong>ile [42] <strong>in</strong> order to illustrate which phase<br />
contributes to <strong>the</strong> buoyancy <strong>of</strong> <strong>the</strong> <strong>subducted</strong><br />
<strong>MORB</strong> <strong>crust</strong> <strong>in</strong> <strong>the</strong> <strong>lower</strong> mantle.<br />
Mg-perovskite is <strong>the</strong> densest phase below 100 GPa<br />
<strong>and</strong> is much denser than <strong>the</strong> mean <strong>lower</strong> mantle (Fig.<br />
5a). The post-perovskite phase is denser than perovskite<br />
by about 3% at <strong>the</strong> phase <strong>transition</strong>. SiO2 phases<br />
are least compressible <strong>and</strong> are <strong>the</strong> lightest m<strong>in</strong>eral <strong>in</strong><br />
<strong>MORB</strong> composition <strong>in</strong> a pressure range studied here<br />
(Fig. 5b). Al-bear<strong>in</strong>g stishovite <strong>and</strong> CaCl2-type SiO2<br />
phase are less dense than <strong>the</strong> PREM <strong>density</strong> at high<br />
temperatures. The <strong>density</strong> a-PbO 2-type SiO 2 phase<br />
strongly depends on <strong>the</strong> substitution mechanism <strong>of</strong><br />
Al 2O 3. Two types <strong>of</strong> mechanism, (1) oxygen vacancytype<br />
<strong>and</strong> (2) octahedral vacancy-occupied-type, are<br />
considered. In both <strong>the</strong> cases, Al-bear<strong>in</strong>g a-PbO2type<br />
SiO2 phase is remarkably less dense than<br />
CaCl2-type phase at equivalent pressure. The <strong>density</strong><br />
<strong>of</strong> SiO2 phase <strong>in</strong> <strong>MORB</strong> composition decreases at <strong>the</strong><br />
phase <strong>transition</strong> to a-PbO 2-type phase due to <strong>the</strong><br />
<strong>in</strong>corporation <strong>of</strong> much higher Al 2O 3 content. a-<br />
PbO 2-type SiO 2 phase significantly contributes to<br />
<strong>the</strong> buoyancy <strong>of</strong> <strong>subducted</strong> <strong>MORB</strong> <strong>crust</strong> <strong>in</strong> <strong>the</strong> <strong>lower</strong>most<br />
mantle. Ca-perovskite is marg<strong>in</strong>ally denser<br />
than <strong>the</strong> mean <strong>lower</strong> mantle (Fig. 5c). The <strong>density</strong><br />
Fig. 5. Density pr<strong>of</strong>ile <strong>of</strong> each constituent m<strong>in</strong>eral <strong>in</strong> <strong>MORB</strong><br />
composition. Two types <strong>of</strong> Al substitution mechanisms <strong>in</strong> a-<br />
PbO2-type SiO2 phase, oxygen vacancy-type (reversed triangles)<br />
<strong>and</strong> octahedral vacancy-occupied-type (squares), are considered<br />
here. O<strong>the</strong>r symbols are same as those <strong>in</strong> Fig. 4. The PREM <strong>density</strong><br />
is shown for comparison by broken l<strong>in</strong>es [42]. High-temperature<br />
(2070–2410 K) data by Ono et al. [7] are also plotted by pluses. The<br />
ma<strong>in</strong> difference is seen <strong>in</strong> <strong>the</strong> <strong>density</strong> pr<strong>of</strong>ile <strong>of</strong> CaFe2O4-type Al<br />
phase, which is primarily due to <strong>the</strong> difference <strong>in</strong> chemical composition,<br />
especially <strong>in</strong> FeO content.