E. Murad, D-95615 Marktredwitz, Germany
E. Murad, D-95615 Marktredwitz, Germany
E. Murad, D-95615 Marktredwitz, Germany
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Martian Phyllosilicates: Recorders of Aqueous Processes (2008) 7020.pdf<br />
Transmission (%)<br />
100<br />
98<br />
96<br />
100<br />
99<br />
100<br />
98<br />
96<br />
94<br />
92<br />
Nontronite<br />
Kaolinite<br />
Illite<br />
-5 -2.5 0<br />
Velocity (mm/s)<br />
2.5 5<br />
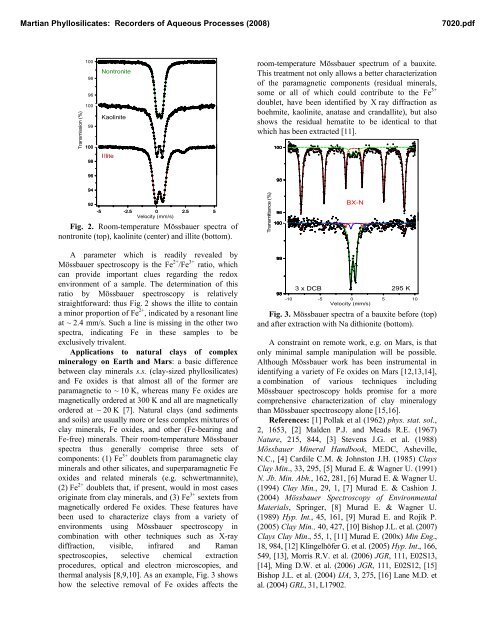
Fig. 2. Room-temperature Mössbauer spectra of<br />
nontronite (top), kaolinite (center) and illite (bottom).<br />
A parameter which is readily revealed by<br />
Mössbauer spectroscopy is the Fe 2+ /Fe 3+ ratio, which<br />
can provide important clues regarding the redox<br />
environment of a sample. The determination of this<br />
ratio by Mössbauer spectroscopy is relatively<br />
straightforward: thus Fig. 2 shows the illite to contain<br />
a minor proportion of Fe 2+ , indicated by a resonant line<br />
at ~ 2.4 mm/s. Such a line is missing in the other two<br />
spectra, indicating Fe in these samples to be<br />
exclusively trivalent.<br />
Applications to natural clays of complex<br />
mineralogy on Earth and Mars: a basic difference<br />
between clay minerals s.s. (clay-sized phyllosilicates)<br />
and Fe oxides is that almost all of the former are<br />
paramagnetic to ~ 10 K, whereas many Fe oxides are<br />
magnetically ordered at 300 K and all are magnetically<br />
ordered at ~ 20 K [7]. Natural clays (and sediments<br />
and soils) are usually more or less complex mixtures of<br />
clay minerals, Fe oxides, and other (Fe-bearing and<br />
Fe-free) minerals. Their room-temperature Mössbauer<br />
spectra thus generally comprise three sets of<br />
components: (1) Fe 3+ doublets from paramagnetic clay<br />
minerals and other silicates, and superparamagnetic Fe<br />
oxides and related minerals (e.g. schwertmannite),<br />
(2) Fe 2+ doublets that, if present, would in most cases<br />
originate from clay minerals, and (3) Fe 3+ sextets from<br />
magnetically ordered Fe oxides. These features have<br />
been used to characterize clays from a variety of<br />
environments using Mössbauer spectroscopy in<br />
combination with other techniques such as X-ray<br />
diffraction, visible, infrared and Raman<br />
spectroscopies, selective chemical extraction<br />
procedures, optical and electron microscopies, and<br />
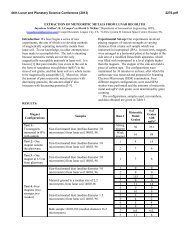
thermal analysis [8,9,10]. As an example, Fig. 3 shows<br />
how the selective removal of Fe oxides affects the<br />
room-temperature Mössbauer spectrum of a bauxite.<br />
This treatment not only allows a better characterization<br />
of the paramagnetic components (residual minerals,<br />
some or all of which could contribute to the Fe 3+<br />
doublet, have been identified by X ray diffraction as<br />
boehmite, kaolinite, anatase and crandallite), but also<br />
shows the residual hematite to be identical to that<br />
which has been extracted [11].<br />
Transmittance (%)<br />
100<br />
98<br />
96<br />
100<br />
99<br />
98<br />
BX-N<br />
3 x DCB 295 K<br />
-10 -5 0 5 10<br />
Velocity (mm/s)<br />
Fig. 3. Mössbauer spectra of a bauxite before (top)<br />
and after extraction with Na dithionite (bottom).<br />
A constraint on remote work, e.g. on Mars, is that<br />
only minimal sample manipulation will be possible.<br />
Although Mössbauer work has been instrumental in<br />
identifying a variety of Fe oxides on Mars [12,13,14],<br />
a combination of various techniques including<br />
Mössbauer spectroscopy holds promise for a more<br />
comprehensive characterization of clay mineralogy<br />
than Mössbauer spectroscopy alone [15,16].<br />
References: [1] Pollak et al (1962) phys. stat. sol.,<br />
2, 1653, [2] Malden P.J. and Meads R.E. (1967)<br />
Nature, 215, 844, [3] Stevens J.G. et al. (1988)<br />
Mössbauer Mineral Handbook, MEDC, Asheville,<br />
N.C., [4] Cardile C.M. & Johnston J.H. (1985) Clays<br />
Clay Min., 33, 295, [5] <strong>Murad</strong> E. & Wagner U. (1991)<br />
N. Jb. Min. Abh., 162, 281, [6] <strong>Murad</strong> E. & Wagner U.<br />
(1994) Clay Min., 29, 1, [7] <strong>Murad</strong> E. & Cashion J.<br />
(2004) Mössbauer Spectroscopy of Environmental<br />
Materials, Springer, [8] <strong>Murad</strong> E. & Wagner U.<br />
(1989) Hyp. Int., 45, 161, [9] <strong>Murad</strong> E. and Rojík P.<br />
(2005) Clay Min., 40, 427, [10] Bishop J.L. et al. (2007)<br />
Clays Clay Min., 55, 1, [11] <strong>Murad</strong> E. (200x) Min Eng.,<br />
18, 984, [12] Klingelhöfer G. et al. (2005) Hyp. Int., 166,<br />
549, [13], Morris R.V. et al. (2006) JGR, 111, E02S13,<br />
[14], Ming D.W. et al. (2006) JGR, 111, E02S12, [15]<br />
Bishop J.L. et al. (2004) IJA, 3, 275, [16] Lane M.D. et<br />
al. (2004) GRL, 31, L17902.