PureProteome Crosslink Antibodies - Millipore
PureProteome Crosslink Antibodies - Millipore
PureProteome Crosslink Antibodies - Millipore
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Protocol guide<br />
Immunoprecipitation by<br />
crosslinking antibodies to<br />
<strong>PureProteome</strong> Protein A, G<br />
or A/G mix magnetic beads<br />
<strong>Crosslink</strong>ing immunoprecipitation:<br />
antigen elution without antibody<br />
interference<br />
Protein A and G magnetic beads are routinely used in<br />
immunoprecipitation (IP) experiments. In traditional IP,<br />
the capture antibody binds reversibly to immobilized<br />
protein A or G and can be easily eluted along with<br />
the target using pH changes or denaturing elution<br />
conditions. In some instances, this coelution of the<br />
capture antibody can interfere with immunodetection,<br />
when the antibody’s heavy or light chain obscures<br />
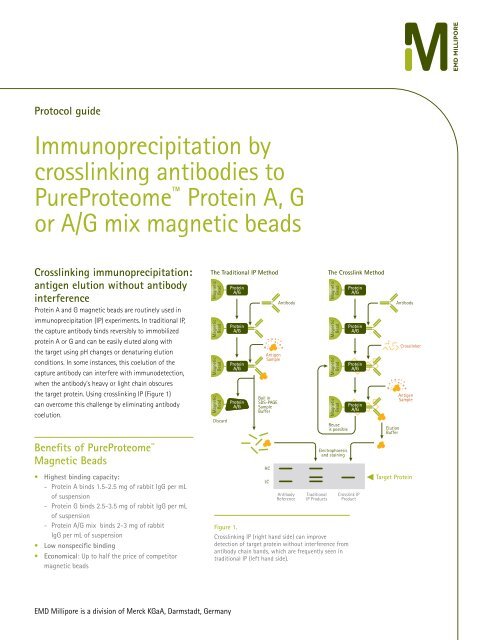
the target protein. Using crosslinking IP (Figure 1)<br />
can overcome this challenge by eliminating antibody<br />
coelution.<br />
The Traditional IP Method<br />
Magnetic<br />
Bead<br />
Magnetic<br />
Bead<br />
Magnetic<br />
Bead<br />
Magnetic<br />
Bead<br />
Discard<br />
Protein<br />
A/G<br />
Protein<br />
A/G<br />
Protein<br />
A/G<br />
Protein<br />
A/G<br />
Antigen<br />
Sample<br />
Boil in<br />
SDS-PAGE<br />
Sample<br />
Buffer<br />
Antibody<br />
The <strong>Crosslink</strong> Method<br />
Magnetic<br />
Bead<br />
Magnetic<br />
Bead<br />
Magnetic<br />
Bead<br />
Magnetic<br />
Bead<br />
Reuse<br />
is possible<br />
Protein<br />
A/G<br />
Protein<br />
A/G<br />
Protein<br />
A/G<br />
Protein<br />
A/G<br />
Elution<br />
Buffer<br />
Antibody<br />
<strong>Crosslink</strong>er<br />
Antigen<br />
Sample<br />
Benefits of <strong>PureProteome</strong> <br />
Magnetic Beads<br />
• Highest binding capacity:<br />
- Protein A binds 1.5-2.5 mg of rabbit IgG per mL<br />
of suspension<br />
- Protein G binds 2.5-3.5 mg of rabbit IgG per mL<br />
of suspension<br />
- Protein A/G mix binds 2-3 mg of rabbit<br />
IgG per mL of suspension<br />
• Low nonspecific binding<br />
• Economical: Up to half the price of competitor<br />
magnetic beads<br />
HC<br />
LC<br />
Antibody<br />
Reference<br />
Traditional<br />
IP Products<br />
Electrophoresis<br />
and staining<br />
<strong>Crosslink</strong> IP<br />
Product<br />
Figure 1.<br />
<strong>Crosslink</strong>ing IP (right hand side) can improve<br />
detection of target protein without interference from<br />
antibody chain bands, which are frequently seen in<br />
traditional IP (left hand side).<br />
Target Protein<br />
EMD <strong>Millipore</strong> is a division of Merck KGaA, Darmstadt, Germany
To avoid coelution, the capture antibody may be<br />
crosslinked to the protein A or G immobilized on the<br />
<strong>PureProteome</strong> magnetic beads. Commonly used<br />
crosslinking agents include BS 3 (Bis(sulfosuccinimidyl)<br />
suberate), DSS (disuccinimidyl suberate) and DMP<br />
(dimethyl pimelimidate). These homobifunctional aminereactive<br />
crosslinkers react with the primary amines<br />
of the immobilized protein A or G and the capture<br />
antibody, forming a stable amide bond. While BS 3 and<br />
DMP are water-soluble, DSS has to be initially dissolved<br />
in dimethyl sulfoxide (DMSO) or dimethyl formamide<br />
(DMF); once dissolved it can be diluted to the working<br />
concentration using a non-amine-containing buffer.<br />
Here, we describe strategies for covalent crosslinking of<br />
antibody to the <strong>PureProteome</strong> protein A, G or A/G mix<br />
magnetic mix beads. The methods provided are guidelines<br />
and may be further optimized for individual applications.<br />
Materials<br />
<strong>PureProteome</strong> Protein A Magnetic Beads,<br />
Catalogue No. LSKMAGA10 or LSKMAGA02<br />
<strong>PureProteome</strong> Protein G Magnetic Beads,<br />
Catalogue No. LSKMAGG10 or LSKMAGG02<br />
<strong>PureProteome</strong> Protein A/G Mix Magnetic Beads,<br />
Catalogue No. LSKMAGAG10 or LSKMAGAG02<br />
<strong>PureProteome</strong> Magnetic Stand,<br />
Catalogue No. LSKMAGS08<br />
Antibody of choice (typically 4-10 µg per reaction)<br />
Coupling buffer for crosslinking with BS 3 and DSS:<br />
20 mM sodium phosphate<br />
0.15 M NaCl (pH should be adjusted to 7-9)<br />
Coupling buffer for crosslinking with DMP:<br />
0.2 M triethanolamine pH 8.2-9<br />
Wash/storage buffer (PBS-T):<br />
Phosphate-buffered saline (PBS) pH 7.4<br />
0.01-0.05% Tween® surfactant 20<br />
Quench buffer:<br />
1M Tris HCl pH 7.5<br />
<strong>Crosslink</strong>er:<br />
BS 3 bis(sulfosuccinimidyl) suberate, ProteoChem<br />
Catalogue No. c1103-100mg or equivalent<br />
DSS (disuccinimidyl suberate,) ProteoChem<br />
Catalogue No. c1105-1gm or equivalent<br />
DMP (Dimethyl pimelimidate),<br />
Sigma Catalogue No. D-8388 or equivalent<br />
Sample Results<br />
<strong>Crosslink</strong>ing of antibody to <strong>PureProteome</strong><br />
magnetic beads improve IP results without<br />
antibody contamination<br />
Figure 2 shows that compared to results of standard IP<br />
(“Std. IP”), immunoprecipitation using <strong>PureProteome</strong><br />
protein G magnetic beads crosslinked to anti-GAPDH<br />
results in a single, clear signal corresponding to the<br />
protein of interest. Standard IP lanes also show signals<br />
corresponding to the antibody heavy and light chains. The<br />
bottom panel of Figure 2 shows that, although the ERK1/2<br />
protein is abundant in the lysate input, no ERK1/2 signal<br />
is seen in immunoprecipitated complexes, indicating that<br />
<strong>PureProteome</strong> protein G magnetic beads demonstrate<br />
low nonspecific binding.<br />
A<br />
B<br />
Lysate BS 3 DSS DMP<br />
Std. IP<br />
Std. IP<br />
Std. IP<br />
Bead +<br />
Antibody<br />
No IP<br />
IgG<br />
Heavy<br />
Chain<br />
GAPDH<br />
IgG<br />
Light<br />
Chain<br />
ERK1/2<br />
Figure 2.<br />
Immunoprecipitation of GAPDH from Jurkat cell lysate<br />
using <strong>PureProteome</strong> protein G magnetic beads crosslinked<br />
to the anti-GAPDH capture antibody using either BS 3 ,<br />
DSS or DMP crosslinking agents (A). Immunoprecipitated<br />
complexes from (A) were probed with anti-ERK1/2 to assess<br />
nonspecific binding of proteins to <strong>PureProteome</strong> protein G<br />
magnetic beads (B).<br />
2
Protocol 1<br />
Antibody binding to<br />
<strong>PureProteome</strong> protein A, G or<br />
A/G mix magnetic beads<br />
(for more detailed instructions refer to user guide)<br />
1. Resuspend magnetic beads by vortexing, ensuring that all<br />
of the beads are uniformly resuspended.<br />
2. Pipette 25-50 µL of bead slurry into a 1.5 mL<br />
microcentrifuge tube. Place tube into magnetic stand to<br />
capture the beads. Remove storage buffer with pipette<br />
and discard.<br />
3. Disengage the magnet from the stand and add 500 µL<br />
wash buffer to the beads. Vortex for 10 seconds. Reengage<br />
magnet to capture the beads and discard wash<br />
buffer. Repeat two more times.<br />
4. Disengage the magnet and resuspend the washed beads<br />
in 100 μL PBS-T. Add capture antibody (typically 4-10 μg)<br />
to the resuspended beads.<br />
5. Allow antibody to bind to beads for 10-30 minutes at<br />
room temperature with continuous mixing. If performing<br />
this step at 4 °C, allow antibody to bind for 60 minutes.<br />
6. Re-engage the magnet to capture the beads. Remove the<br />
buffer containing unbound antibody; if necessary, retain<br />
for later analysis.<br />
7. Disengage magnet. Wash beads by adding 500 µL PBS-T,<br />
vortex beads for 10 seconds. Repeat 2 more times for a<br />
total of 3 washes.<br />
8. Engage the magnet to capture beads, remove PBS-T, then<br />
add 500 µL coupling buffer. Remove the magnet and<br />
vortex beads for 60 seconds. Insert magnet, collect beads<br />
and discard buffer. Repeat two more times.<br />
9. Beads are ready for crosslinking.<br />
Protocol 2<br />
Antibody crosslinking using BS 3<br />
Note: <strong>Crosslink</strong>er solution should be prepared immediately<br />
before use and should not be stored for later use.<br />
1. Each crosslinking reaction requires 250 µL of 5 mM<br />
crosslinker solution.<br />
2. Prepare a 100 mM BS 3 stock solution by weighing out<br />
2 mg of BS 3 and dissolving in 35 µL Milli-Q® water.<br />
3. Once dissolved, dilute to 5 mM by adding 665 µL<br />
coupling buffer.<br />
4. Add 250 µL of 5 mM BS 3 solution to each crosslinking<br />
reaction and incubate with end-over-end mixing for 30-<br />
60 minutes at room temperature.<br />
5. To quench the crosslinker, add 12.5 µL of quench buffer<br />
to each reaction, incubate for 30-60 minutes at room<br />
temperature with end-over-end mixing.<br />
6. Engage magnet to capture the beads, then remove the<br />
solution with a pipette and discard. Disengage magnet.<br />
7. Wash beads 1x 500 µL for 1 minute using 0.2 M glycine<br />
HCl, pH 2.5 to remove any non-crosslinked antibody.<br />
Engage magnet to capture beads, then remove glycine<br />
solution with pipette and discard.<br />
8. Wash beads for 1 minute using 500 µL PBS-T.<br />
Repeat 2 more times for a total of 3 washes.<br />
9. Use beads immediately in IP experiments or store at<br />
4 °C. For long-term storage, it is recommended that a<br />
bacteriostat such as sodium azide (0.05%) is added to the<br />
bead storage buffer to prevent microbial growth.<br />
Protocol 3<br />
Antibody crosslinking using DSS<br />
Note: <strong>Crosslink</strong>er solution should be prepared immediately<br />
before use and should not be stored for later use.<br />
1. Each crosslinking reaction requires 250 µL of 5 mM<br />
crosslinker solution.<br />
2. Prepare a 100 mM DSS stock solution by weighing out<br />
2 mg of DSS and dissolving in 54 µL DMSO or DMF.<br />
3. Once dissolved, dilute to 5 mM by adding 646 µL<br />
coupling buffer.<br />
4. Add 250 µL of 5 mM DSS solution to each crosslinking<br />
reaction and incubate with end-over-end mixing for<br />
30-60 minutes at room temperature.<br />
5. To quench the crosslinker, add 12.5 µL of quench buffer to<br />
each reaction and incubate for 30-60 minutes at room<br />
temperature with end-over-end mixing.<br />
6. Place tube into magnetic stand to capture beads, remove<br />
solution with a pipette and discard.<br />
7. Wash beads for 1 minute with 500 μL 0.2 M glycine HCl,<br />
pH 2.5 to remove any non-crosslinked antibody. Engage<br />
magnet to capture beads and discard solution.<br />
8. Wash beads for 1 minute using 500 µL PBS-T. Repeat 2 more<br />
times for a total of 3 washes.<br />
9. Use beads immediately in IP experiments or store at 4 °C.<br />
For long-term storage, it is recommended that a bacteriostat<br />
such as sodium azide (0.05%) is added to the bead storage<br />
buffer to prevent microbial growth.<br />
Protocol 4<br />
Antibody crosslinking using DMP<br />
Note: <strong>Crosslink</strong>er solution should be prepared immediately<br />
before use and should not be stored for later use.<br />
1. Each crosslinking reaction requires 500 µL of 20 mM<br />
crosslinker solution.<br />
2. Prepare 20 mM DMP solution by weighing out 4 mg DMP<br />
and dissolving in 772 µL of 0.2 M triethanolamine coupling<br />
buffer.<br />
3. Add 500 µL of 20 mM DMP solution to each crosslinking<br />
reaction and incubate with end-over-end mixing for<br />
60 minutes at room temperature.<br />
4. To quench the crosslinker, add 50 µL of quench buffer to<br />
each reaction and incubate for 30-60 minutes at room<br />
temperature with end-over-end mixing.<br />
5. Place tube into magnetic stand, collect beads and remove<br />
solution.<br />
6. Wash beads for 1 minute with 500 μL 0.2 M glycine HCl, pH<br />
2.5 to remove any non-crosslinked antibody. Engage magnet<br />
to capture beads and discard solution.<br />
7. Wash beads for 1 minute using 500 μL PBS-T.<br />
Repeat 2 more times for a total of 3 washes.<br />
8. Use beads immediately in IP experiments or store at<br />
4 °C. For long-term storage, it is recommended that a<br />
bacteriostat such as sodium azide (0.05%) is added to the<br />
bead storage buffer to prevent microbial growth.<br />
3
Figure 3.<br />
The <strong>PureProteome</strong><br />
Magnetic Stand is<br />
designed to rapidly and<br />
easily isolate magnetic<br />
particles from up to eight<br />
1.5 mL or 2.0 mL tubes.<br />
The stand features a<br />
removable magnet and<br />
unique vortex interface<br />
that enables thorough<br />
mixing without having to<br />
remove tubes from the<br />
stand.<br />
Ordering Information<br />
Description Qty/Pk Catalogue No.<br />
<strong>PureProteome</strong> Protein A Magnetic Beads 2 x 1 mL LSKMAGA02<br />
1 x 10 mL LSKMAGA10<br />
<strong>PureProteome</strong> Protein G Magnetic Beads 2 x 1 mL LSKMAGG02<br />
1 x 10 mL LSKMAGG10<br />
<strong>PureProteome</strong> Protein A/G Mix Magnetic Beads 2 x 1 mL LSKMAGAG02<br />
1 x 10 mL LSKMAGAG10<br />
<strong>PureProteome</strong> Magnetic Stand, 8-well 1 LSKMAGS08<br />
To Place an Order or Receive<br />
Technical Assistance<br />
In the U.S. and Canada, call toll-free 1-800-645-5476<br />
For other countries across Europe and the world,<br />
please visit: www.emdmillipore.com/offices<br />
For Technical Service, please visit:<br />
www.emdmillipore.com/techservice<br />
Get Connected!<br />
Join EMD <strong>Millipore</strong> Bioscience on your favorite social<br />
media outlet for the latest updates, news, products,<br />
innovations, and contests!<br />
www.emdmillipore.com/offices<br />
facebook.com/EMD<strong>Millipore</strong>Bioscience<br />
twitter.com/EMD<strong>Millipore</strong>Bio<br />
EMD <strong>Millipore</strong>, the M logo, and <strong>PureProteome</strong> are trademarks<br />
and Milli-Q is a registered trademark of Merck KGaA, Darmstadt, Germany.<br />
Trademarks belonging to third parties are the properties of their respective owners.<br />
Lit No. PC5522EN00 BS-GEN-13-07871 03/2013 Printed in the USA.<br />
© 2013 EMD <strong>Millipore</strong> Corporation, Billerica, MA USA. All rights reserved.