Abstracts of the Scientific Posters, 2013 AACC Annual Meeting ...
Abstracts of the Scientific Posters, 2013 AACC Annual Meeting ...
Abstracts of the Scientific Posters, 2013 AACC Annual Meeting ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Technology/Design Development<br />
Wednesday, July 31, 9:30 am – 5:00 pm<br />
Samples: The serum and plasma samples were collected from inpatients/outpatients<br />
and <strong>the</strong> employees who volunteered. This study has been approved by <strong>the</strong> ethical<br />
committee in Hamamatsu University School <strong>of</strong> Medicine.<br />
Material and Method: The reagent PANACLEAR MMP-3 “Latex” (Sekisui Medical<br />
Co., Ltd.) and <strong>the</strong> automated clinical chemistry analyzer LABOSPECT 008 (Hitachi)<br />
were used for <strong>the</strong> following evaluations: (1) precision, (2) dilution linearity (2 level <strong>of</strong><br />
MMP-3 samples were diluted with physiological saline) and limit <strong>of</strong> detection (2.6SD<br />
method), (3) interference (evaluated with Interference Check (Sysmex) and ascorbic<br />
acid), (4) correlation with results from JCA-BM1650 (JEOL Ltd.), and (5) probe<br />
contamination test (evaluated on 42 parameters <strong>of</strong> <strong>the</strong> same pre-installed module).<br />
Results: (1) Within-run precision <strong>of</strong> CV (n=20) :1.35 % (Control L; mean 110.9 ng/<br />
mL), 0.78 % (Control H; mean 435.4 ng/mL ), 1.62 % (pooled serum; mean 99.2 ng/<br />
mL) (2) Linearity was up to 1500 ng/mL and limit <strong>of</strong> detection was 9.85 ng/mL. (3)<br />
No influences were observed by bilirubin < 200 mg/L, hemoglobin < 5 g/L, RF < 550<br />
U/mL or ascorbic acid < 500 mg/L in sample. (4) High correlation was noted, with <strong>the</strong><br />
regression formula being y = 0.991x-13.9 and <strong>the</strong> correlation coefficient being 0.985<br />
(N=92). (5) Probe contamination test: No influence by prove contamination test was<br />
noted on any <strong>of</strong> <strong>the</strong> 42 parameters.<br />
Conclusion: PANACLEAR MMP-3 “Latex” with LABOSPECT 008 was shown in<br />
<strong>the</strong> present study as having favorable performance. Wide assay range with linearity<br />
up to 1500 ng/ml is especially convenient because high level MMP-3 samples don’t<br />
require dilution and retesting. Additionally, in couple with LABOSPECT 008, routine<br />
measurement <strong>of</strong> MMP-3 could be easily handled, and real-time reporting could lead<br />
to clinical remission. This reagent could be useful for measuring MMP-3 in clinical<br />
laboratories.<br />
B-255<br />
GlycA and GlycB: Novel NMR Markers <strong>of</strong> Systemic Inflammation<br />
J. Otvos, I. Shalaurova, J. Wolak-Dinsmore, S. Matyus. LipoScience,<br />
Raleigh, NC<br />
Background: Serum concentrations <strong>of</strong> many glycosylated acute-phase proteins such<br />
as C-reactive protein (CRP) and fibrinogen are used clinically to assess and monitor<br />
both acute and chronic inflammation. Serum NMR spectra obtained for lipoprotein<br />
particle analysis using <strong>the</strong> automated NMR Pr<strong>of</strong>iler system contain two NMR signals<br />
originating from N-acetyl methyl group protons on <strong>the</strong> N- and O-linked glycans <strong>of</strong><br />
serum glycoproteins. One, that we named GlycA, comes from N-acetylglucosamine<br />
and N-acetylgalactosamine and <strong>the</strong> o<strong>the</strong>r, GlycB, from sialic acid moieties. We<br />
hypo<strong>the</strong>sized that <strong>the</strong> measured amplitudes <strong>of</strong> <strong>the</strong>se signals would reflect global<br />
protein glycosylation levels, <strong>the</strong>reby providing measures <strong>of</strong> inflammation status<br />
that might have clinical utility similar or complementary to existing inflammatory<br />
biomarkers.<br />
Methods: We used archived serum NMR spectra from previously-performed<br />
automated NMR LipoPr<strong>of</strong>ile (lipoprotein particle) analyses conducted using <strong>the</strong> 400<br />
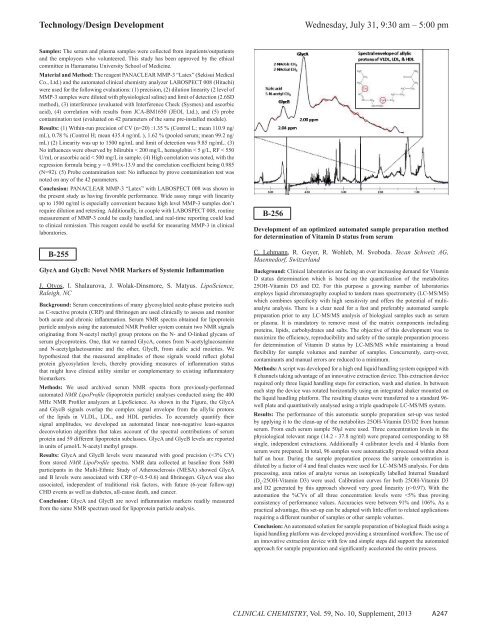
MHz NMR Pr<strong>of</strong>iler analyzers at LipoScience. As shown in <strong>the</strong> Figure, <strong>the</strong> GlycA<br />
and GlycB signals overlap <strong>the</strong> complex signal envelope from <strong>the</strong> allylic protons<br />
<strong>of</strong> <strong>the</strong> lipids in VLDL, LDL, and HDL particles. To accurately quantify <strong>the</strong>ir<br />
signal amplitudes, we developed an automated linear non-negative least-squares<br />
deconvolution algorithm that takes account <strong>of</strong> <strong>the</strong> spectral contributions <strong>of</strong> serum<br />
protein and 59 different lipoprotein subclasses. GlycA and GlycB levels are reported<br />
in units <strong>of</strong> μmol/L N-acetyl methyl groups.<br />
Results: GlycA and GlycB levels were measured with good precision (0.97). With <strong>the</strong><br />
automation <strong>the</strong> %CVs <strong>of</strong> all three concentration levels were