Handbook Part 2 - International Mycological Association
Handbook Part 2 - International Mycological Association
Handbook Part 2 - International Mycological Association
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
PS4-398-0143<br />
Stachylina in India: occurrence and relationships with temperature and hydrogen-ion concentration of the<br />
waters<br />
J. K. Misra<br />
Sri J. N. P. G. College, Lucknow, Uttar Pradesh, India<br />
Bioinventoring of Harpellales (Trichomycetes) was undertaken to fill the lacuna in our knowledge of these fungi from<br />
India.<br />
Bloodworms (Chironomus sp, Chironomidae) were regularly collected for a year (Jan-Dec. 2004) from stagnant water<br />
bodies in and around Lucknow by scooping the muddy water using iron strainer. Temperature and pH of the water<br />
were recorded at the time of collection of bloodworms. The bloodworms were dissected in a drop of distilled water<br />
under binocular stereomicroscope and peritrophic membranes were examined under phase contrast microscope in<br />
water mount for the presence of the thalli of Stachylina on them.<br />
Stachylina chironomidarum and St. grandispora were observed. St. chironomidarum was not common in occurrence<br />
while St. grandispora was found throughout the year, of course, in varying percentages as shown in table below. St.<br />
grandispora exhibited enough variations in the number and size of the trichospores.<br />
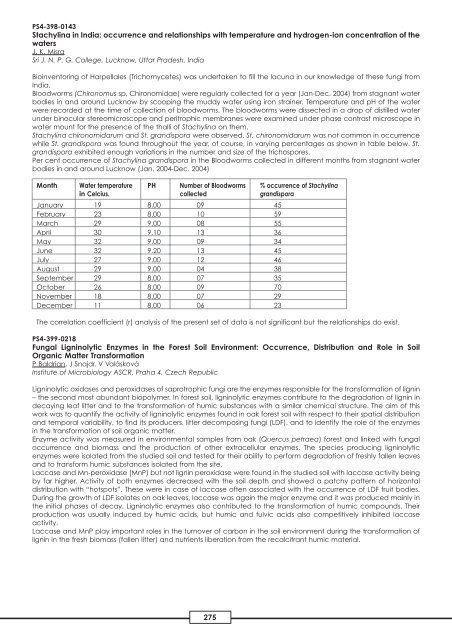
Per cent occurrence of Stachylina grandispora in the Bloodworms collected in different months from stagnant water<br />
bodies in and around Lucknow (Jan. 2004-Dec. 2004)<br />
Month Water temperature PH Number of Bloodworms % occurrence of Stachylina<br />
in Celcius. collected grandispora<br />
January 19 8.00 09 45<br />
February 23 8.00 10 59<br />
March 29 9.00 08 55<br />
April 30 9.10 13 36<br />
May 32 9.00 09 34<br />
June 32 9.20 13 45<br />
July 27 9.00 12 46<br />
August 29 9.00 04 38<br />
September 29 8.00 07 35<br />
October 26 8.00 09 70<br />
November 18 8.00 07 29<br />
December 11 8.00 06 23<br />
The correlation coefficient (r) analysis of the present set of data is not significant but the relationships do exist.<br />
PS4-399-0218<br />
Fungal Ligninolytic Enzymes in the Forest Soil Environment: Occurrence, Distribution and Role in Soil<br />
Organic Matter Transformation<br />
P Baldrian, J Snajdr, V Valásková<br />
Institute of Microbiology ASCR, Praha 4, Czech Republic<br />
Ligninolytic oxidases and peroxidases of saprotrophic fungi are the enzymes responsible for the transformation of lignin<br />
– the second most abundant biopolymer. In forest soil, ligninolytic enzymes contribute to the degradation of lignin in<br />
decaying leaf litter and to the transformation of humic substances with a similar chemical structure. The aim of this<br />
work was to quantify the activity of ligninolytic enzymes found in oak forest soil with respect to their spatial distribution<br />
and temporal variability, to find its producers, litter decomposing fungi (LDF), and to identify the role of the enzymes<br />
in the transformation of soil organic matter.<br />
Enzyme activity was measured in environmental samples from oak (Quercus petraea) forest and linked with fungal<br />
occurrence and biomass and the production of other extracellular enzymes. The species producing ligninolytic<br />
enzymes were isolated from the studied soil and tested for their ability to perform degradation of freshly fallen leaves<br />
and to transform humic substances isolated from the site.<br />
Laccase and Mn-peroxidase (MnP) but not lignin peroxidase were found in the studied soil with laccase activity being<br />
by far higher. Activity of both enzymes decreased with the soil depth and showed a patchy pattern of horizontal<br />
distribution with “hotspots”. These were in case of laccase often associated with the occurrence of LDF fruit bodies.<br />
During the growth of LDF isolates on oak leaves, laccase was again the major enzyme and it was produced mainly in<br />
the initial phases of decay. Ligninolytic enzymes also contributed to the transformation of humic compounds. Their<br />
production was usually induced by humic acids, but humic and fulvic acids also competitively inhibited laccase<br />
activity.<br />
Laccase and MnP play important roles in the turnover of carbon in the soil environment during the transformation of<br />
lignin in the fresh biomass (fallen litter) and nutrients liberation from the recalcitrant humic material.<br />
275