The Global Use of Medicines - IMS Health
The Global Use of Medicines - IMS Health
The Global Use of Medicines - IMS Health
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
POLICY-DRIVEN CHANGES AND THEIR IMPACTS THROUGH 2015<br />
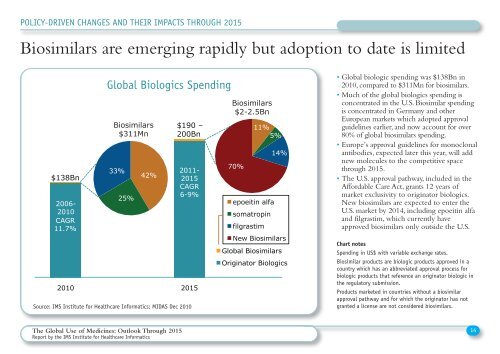
Biosimilars are emerging rapidly but adoption to date is limited<br />
$138Bn<br />
2006-<br />
2010<br />
CAGR<br />
11.7%<br />
<strong>Global</strong> Biologics Spending<br />
2010 2015<br />
Biosimilars<br />
$2-2.5Bn<br />
Biosimilars $190 –<br />
11%<br />
$311Mn 200Bn<br />
5%<br />
33%<br />
25%<br />
42%<br />
2011-<br />
2015<br />
CAGR<br />
6-9%<br />
Source: <strong>IMS</strong> Institute for <strong>Health</strong>care Informatics; MIDAS Dec 2010<br />
70%<br />
epoeitin alfa<br />
somatropin<br />
filgrastim<br />
14%<br />
New Biosimilars<br />
<strong>Global</strong> Biosimilars<br />
Originator Biologics<br />
• <strong>Global</strong> biologic spending was $138Bn in<br />
2010, compared to $311Mn for biosimilars.<br />
• Much <strong>of</strong> the global biologics spending is<br />
concentrated in the U.S. Biosimilar spending<br />
is concentrated in Germany and other<br />
European markets which adopted approval<br />
guidelines earlier, and now account for over<br />
80% <strong>of</strong> global biosimilars spending.<br />
• Europe’s approval guidelines for monoclonal<br />
antibodies, expected later this year, will add<br />
new molecules to the competitive space<br />
through 2015.<br />
• <strong>The</strong> U.S. approval pathway, included in the<br />
Affordable Care Act, grants 12 years <strong>of</strong><br />
market exclusivity to originator biologics.<br />
New biosimilars are expected to enter the<br />
U.S. market by 2014, including epoeitin alfa<br />
and filgrastim, which currently have<br />
approved biosimilars only outside the U.S.<br />
Chart notes<br />
Spending in US$ with variable exchange rates.<br />
Biosimilar products are biologic products approved in a<br />
country which has an abbreviated approval process for<br />
biologic products that reference an originator biologic in<br />
the regulatory submission.<br />
Products marketed in countries without a biosimilar<br />
approval pathway and for which the originator has not<br />
granted a license are not considered biosimilars.<br />
.<br />
<strong>The</strong> <strong>Global</strong> <strong>Use</strong> <strong>of</strong> <strong>Medicines</strong>: Outlook Through 2015<br />
Report by the <strong>IMS</strong> Institute for <strong>Health</strong>care Informatics<br />
14