From vision to decision Pharma 2020 - pwc
From vision to decision Pharma 2020 - pwc
From vision to decision Pharma 2020 - pwc
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Creating companion diagnostics for medicines that target<br />
a specific disease subtype lets doc<strong>to</strong>rs maximise the value<br />
of those medicines themselves<br />
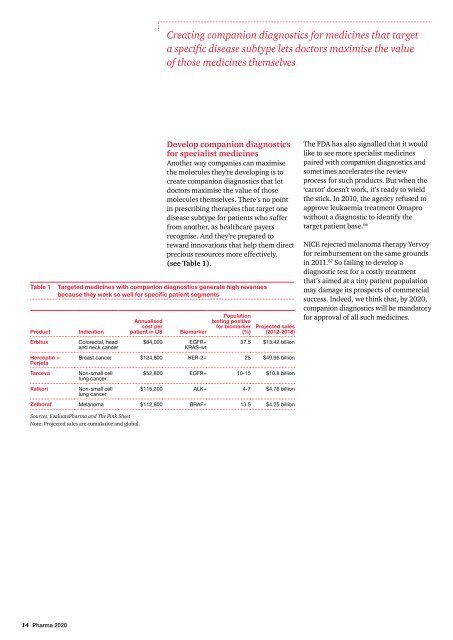
Table 1<br />
Product<br />
Erbitux<br />
Herceptin +<br />
Perjeta<br />
Tarceva<br />
Xalkori<br />
Indication<br />
Colorectal, head<br />
and neck cancer<br />
Annualised<br />
cost per<br />
patient in US<br />
Biomarker<br />
$84,000 EGFR+<br />
KRAS-wt<br />
Population<br />
testing positive<br />
for biomarker<br />
(%)<br />
Projected sales<br />
(2012-2018)<br />
37.5 $13.42 billion<br />
Breast cancer $124,800 HER-2+ 25 $49.96 billion<br />
Non-small cell<br />
lung cancer<br />
Non-small cell<br />
lung cancer<br />
Develop companion diagnostics<br />
for specialist medicines<br />
Another way companies can maximise<br />
the molecules they’re developing is <strong>to</strong><br />
create companion diagnostics that let<br />
doc<strong>to</strong>rs maximise the value of those<br />
molecules themselves. There’s no point<br />
in prescribing therapies that target one<br />
disease subtype for patients who suffer<br />
from another, as healthcare payers<br />
recognise. And they’re prepared <strong>to</strong><br />
reward innovations that help them direct<br />
precious resources more effectively.<br />
(see Table 1).<br />
Targeted medicines with companion diagnostics generate high revenues<br />
because they work so well for specific patient segments<br />
$52,800 EGFR+ 10-15 $10.8 billion<br />
$115,200 ALK+ 4-7 $4.76 billion<br />
Zelboraf Melanoma $112,800 BRAF+ 13.5 $4.25 billion<br />
The FDA has also signalled that it would<br />
like <strong>to</strong> see more specialist medicines<br />
paired with companion diagnostics and<br />
sometimes accelerates the review<br />
process for such products. But when the<br />
‘carrot’ doesn’t work, it’s ready <strong>to</strong> wield<br />
the stick. In 2010, the agency refused <strong>to</strong><br />
approve leukaemia treatment Omapro<br />
without a diagnostic <strong>to</strong> identify the<br />
target patient base. 66<br />
NICE rejected melanoma therapy Yervoy<br />
for reimbursement on the same grounds<br />
in 2011. 67 So failing <strong>to</strong> develop a<br />
diagnostic test for a costly treatment<br />
that’s aimed at a tiny patient population<br />
may damage its prospects of commercial<br />
success. Indeed, we think that, by <strong>2020</strong>,<br />
companion diagnostics will be manda<strong>to</strong>ry<br />
for approval of all such medicines.<br />
Sources: Evaluate<strong>Pharma</strong> and The Pink Sheet<br />
Note: Projected sales are cumulative and global.<br />
14 <strong>Pharma</strong> <strong>2020</strong>