full text - Universität zu Köln
full text - Universität zu Köln
full text - Universität zu Köln
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
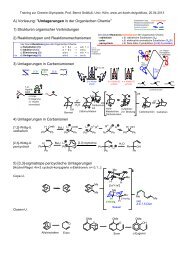
1 Introduction<br />
1.4 Aim of this project<br />
Protein O-mannosylation is a rare posttranslational modification in mammals in<br />
general but was found in about one third of all O-glycosidically bound glycans in the<br />
brain. So far, only very few proteins have been identified to carry this modification<br />
and their O-mannosyl glycans alone cannot account for the high amount present in<br />
nervous tissues. In addition, patients suffering from genetic mutations of enzymes<br />
involved in the O-mannosylation pathway exhibit a severe neurological phenotype<br />
which indicates a still unknown but important role for O-mannosylation in the brain.<br />
Therefore, the aim of this study was the identification of new O-mannosylated<br />
proteins of the mammalian brain leading to new insights into the mechanism of<br />
initiation and the pathology of O-mannosylation defects.<br />
The isolation of O-mannosylated proteins ought to be accomplished from mouse and<br />
calf brains by means of general proteomics. Since the exposed glycan epitopes of<br />
mannose-based glycans are very similar to other N- and O-glycan epitopes immune<br />
affinity purification of O-mannosylated proteins was not possible. Therefore, a broad,<br />
unbiased proteomics approach was chosen in which mouse or calf brain lysates were<br />
to be fractionated via a newly developed sequence of preparative fractionation<br />
methods. The generated protein fractions should be analyzed regarding their<br />
O-glycan content by MALDI mass spectrometry of released O-glycans and proteins<br />
ought to be identified by LC-MS of tryptic peptides. Further information about the<br />
O-mannosylation sites was to be gained from glycopeptide analysis via LC-ESI-MS.<br />
18