full text - Universität zu Köln
full text - Universität zu Köln
full text - Universität zu Köln
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3 Results<br />
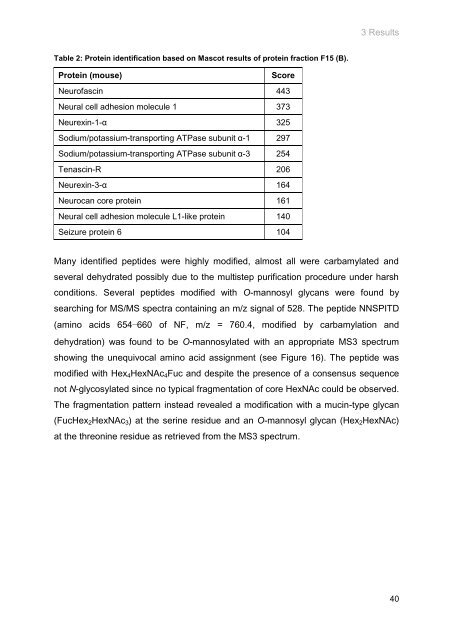
Table 2: Protein identification based on Mascot results of protein fraction F15 (B).<br />
Protein (mouse)<br />
Score<br />
Neurofascin 443<br />
Neural cell adhesion molecule 1 373<br />
Neurexin-1-α 325<br />
Sodium/potassium-transporting ATPase subunit α-1 297<br />
Sodium/potassium-transporting ATPase subunit α-3 254<br />
Tenascin-R 206<br />
Neurexin-3-α 164<br />
Neurocan core protein 161<br />
Neural cell adhesion molecule L1-like protein 140<br />
Sei<strong>zu</strong>re protein 6 104<br />
Many identified peptides were highly modified, almost all were carbamylated and<br />
several dehydrated possibly due to the multistep purification procedure under harsh<br />
conditions. Several peptides modified with O-mannosyl glycans were found by<br />
searching for MS/MS spectra containing an m/z signal of 528. The peptide NNSPITD<br />
(amino acids 654−660 of NF, m/z = 760.4, modified by carbamylation and<br />
dehydration) was found to be O-mannosylated with an appropriate MS3 spectrum<br />
showing the unequivocal amino acid assignment (see Figure 16). The peptide was<br />
modified with Hex 4 HexNAc 4 Fuc and despite the presence of a consensus sequence<br />
not N-glycosylated since no typical fragmentation of core HexNAc could be observed.<br />
The fragmentation pattern instead revealed a modification with a mucin-type glycan<br />
(FucHex 2 HexNAc 3 ) at the serine residue and an O-mannosyl glycan (Hex 2 HexNAc)<br />
at the threonine residue as retrieved from the MS3 spectrum.<br />
40