Review of FDA warning letters for microbial bioburden issues (2001 ...
Review of FDA warning letters for microbial bioburden issues (2001 ...
Review of FDA warning letters for microbial bioburden issues (2001 ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Article<br />
<strong>Review</strong> <strong>of</strong> <strong>FDA</strong> <strong>warning</strong> <strong>letters</strong> <strong>for</strong> <strong>microbial</strong><br />
<strong>bioburden</strong> <strong>issues</strong> (<strong>2001</strong>-2011)<br />
*Dr. Tim Sandle<br />
Head <strong>of</strong> Microbiology, BPL, UK<br />
The monitoring <strong>of</strong> <strong>bioburden</strong>, either as a product being<br />
manufactured, or prior to sterilization (be that through a sterilizing<br />
grade filter or through a terminal sterilization process) is an issue<br />
<strong>of</strong> <strong>FDA</strong> concern.<br />
A review <strong>of</strong> <strong>FDA</strong> <strong>warning</strong> <strong>letters</strong> between <strong>2001</strong> and 2011<br />
indicates that seventy-nine companies have received a <strong>warning</strong><br />
letter observation relating to <strong>bioburden</strong> testing. With some<br />
companies, multiple observations have been given within the same<br />
<strong>warning</strong> letter and 111 observations have been made in relation to<br />
<strong>bioburden</strong> testing <strong>for</strong> this period.<br />
The majority <strong>of</strong> the observations were made during the period<br />
2004-2007, as indicated in the graph below. However, a number <strong>of</strong><br />
<strong>warning</strong> letter observations continues to be issued each year with<br />
the current rate averaging at ten per year.<br />
Figure 1: Graph displaying the number <strong>of</strong> <strong>warning</strong> <strong>letters</strong><br />
which mention a <strong>bioburden</strong> issue <strong>for</strong> the period <strong>2001</strong>-211.<br />
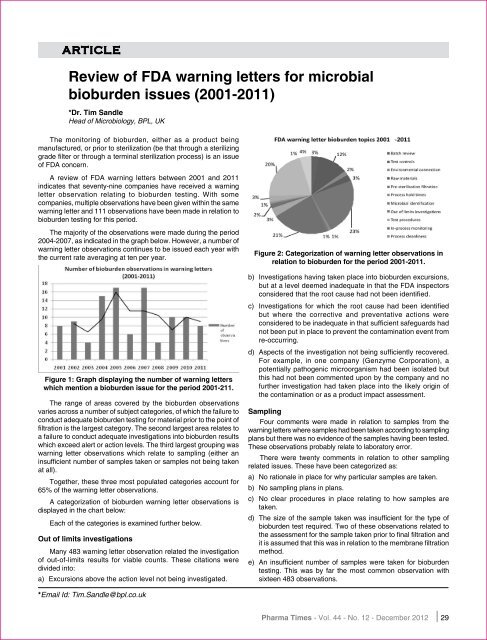
The range <strong>of</strong> areas covered by the <strong>bioburden</strong> observations<br />
varies across a number <strong>of</strong> subject categories, <strong>of</strong> which the failure to<br />
conduct adequate <strong>bioburden</strong> testing <strong>for</strong> material prior to the point <strong>of</strong><br />
filtration is the largest category. The second largest area relates to<br />
a failure to conduct adequate investigations into <strong>bioburden</strong> results<br />
which exceed alert or action levels. The third largest grouping was<br />
<strong>warning</strong> letter observations which relate to sampling (either an<br />
insufficient number <strong>of</strong> samples taken or samples not being taken<br />
at all).<br />
Together, these three most populated categories account <strong>for</strong><br />
65% <strong>of</strong> the <strong>warning</strong> letter observations.<br />
A categorization <strong>of</strong> <strong>bioburden</strong> <strong>warning</strong> letter observations is<br />
displayed in the chart below:<br />
Each <strong>of</strong> the categories is examined further below.<br />
Out <strong>of</strong> limits investigations<br />
Many 483 <strong>warning</strong> letter observation related the investigation<br />
<strong>of</strong> out-<strong>of</strong>-limits results <strong>for</strong> viable counts. These citations were<br />
divided into:<br />
a) Excursions above the action level not being investigated.<br />
Figure 2: Categorization <strong>of</strong> <strong>warning</strong> letter observations in<br />
relation to <strong>bioburden</strong> <strong>for</strong> the period <strong>2001</strong>-2011.<br />
b) Investigations having taken place into <strong>bioburden</strong> excursions,<br />
but at a level deemed inadequate in that the <strong>FDA</strong> inspectors<br />
considered that the root cause had not been identified.<br />
c) Investigations <strong>for</strong> which the root cause had been identified<br />
but where the corrective and preventative actions were<br />
considered to be inadequate in that sufficient safeguards had<br />
not been put in place to prevent the contamination event from<br />
re-occurring.<br />
d) Aspects <strong>of</strong> the investigation not being sufficiently recovered.<br />
For example, in one company (Genzyme Corporation), a<br />
potentially pathogenic microorganism had been isolated but<br />
this had not been commented upon by the company and no<br />
further investigation had taken place into the likely origin <strong>of</strong><br />
the contamination or as a product impact assessment.<br />
Sampling<br />
Four comments were made in relation to samples from the<br />
<strong>warning</strong> <strong>letters</strong> where samples had been taken according to sampling<br />
plans but there was no evidence <strong>of</strong> the samples having been tested.<br />
These observations probably relate to laboratory error.<br />
There were twenty comments in relation to other sampling<br />
related <strong>issues</strong>. These have been categorized as:<br />
a) No rationale in place <strong>for</strong> why particular samples are taken.<br />
b) No sampling plans in plans.<br />
c) No clear procedures in place relating to how samples are<br />
taken.<br />
d) The size <strong>of</strong> the sample taken was insufficient <strong>for</strong> the type <strong>of</strong><br />
<strong>bioburden</strong> test required. Two <strong>of</strong> these observations related to<br />
the assessment <strong>for</strong> the sample taken prior to final filtration and<br />
it is assumed that this was in relation to the membrane filtration<br />
method.<br />
e) An insufficient number <strong>of</strong> samples were taken <strong>for</strong> <strong>bioburden</strong><br />
testing. This was by far the most common observation with<br />
sixteen 483 observations.<br />
*Email Id: tim.sandle@bpl.co.uk<br />
Pharma Times - Vol. 44 - No. 12 - December 2012 29
Pre-sterilization <strong>bioburden</strong><br />
Observations relating to pre-sterilization <strong>bioburden</strong> related<br />
to product subject to terminal sterilization processes. For such<br />
processes there is a requirement to periodically assess the typical<br />
<strong>bioburden</strong> levels on the product and to characterize the types <strong>of</strong><br />
microorganisms recovered.<br />
The observations made in relation to such testing fell into the<br />
following categories:<br />
a) Bioburden levels not assessed.<br />
b) Bioburden levels not assessed periodically (in most cases<br />
the failure to undertaken such an assessment quarterly was<br />
cited).<br />
c) The only comment which slightly differed to the quarterly<br />
required related to a failure to determine <strong>of</strong> the <strong>bioburden</strong> on<br />
the pre-sterilized product varied seasonally.<br />
d) Observations were made in relation to a failure to characterize<br />
the microorganisms detected in terms <strong>of</strong> their theoretical<br />
resistance to the sterilization process.<br />
e) Two observations related to a failure to relate the <strong>bioburden</strong><br />
assessments back to the sterilization validation and a failure to<br />
undertaken a re-validation exercise.<br />
Test controls<br />
Warning <strong>letters</strong> which in relation to test controls were primarily<br />
concerned with in-process samples being tested and reported<br />
without any alert or action levels being in place. The absence <strong>of</strong><br />
any acceptance criteria made the review <strong>of</strong> the intermediate product<br />
meaningless.<br />
A second criticism was that where alert and action levels were<br />
in place, no rationale was available to describe how the alert and<br />
action levels had been set. For alert and action levels, it is common<br />
to either assign these based on one <strong>of</strong> the following:<br />
a) Regulatory guidance values.<br />
b) Based on some measure <strong>of</strong> risk (where a <strong>bioburden</strong> above a<br />
certain threshold could lead to final product contamination).<br />
c) A statistical analysis <strong>of</strong> past data.<br />
A third area to which the <strong>warning</strong> <strong>letters</strong> related was where<br />
action levels had been set but had then been increased without<br />
any explanation or justification provided <strong>for</strong> the increase.<br />
A fourth area, which related to one <strong>warning</strong> letter, was that<br />
the company (Genzyme Corporation), had used the term “Too<br />
Numerous To Count” (TNTC) <strong>for</strong> test data without defining what<br />
TNTC meant and what the implications <strong>of</strong> a result which was<br />
deemed to be TNTC meant.<br />
Test method validation<br />
Five <strong>of</strong> the 483 observations related to test method validation.<br />
In four <strong>of</strong> the cases the <strong>warning</strong> <strong>letters</strong> related to absence <strong>of</strong> test<br />
method validation <strong>for</strong> different <strong>bioburden</strong> samples in that the test<br />
methods used did not show recovery <strong>of</strong> microorganisms.<br />
The fifth observation related to a company which had used a<br />
contract test facility to conduct <strong>bioburden</strong> testing. Here, there was no<br />
evidence <strong>of</strong> method transfer or with the contract laboratory having<br />
conducted validation studies.<br />
Test procedures<br />
There were four 483 observations relating to test procedures.<br />
These concerned either the lack <strong>of</strong> procedures in place (that is,<br />
there was no description <strong>of</strong> how the <strong>bioburden</strong> test was conducted)<br />
or that the procedure was unclear. The lack <strong>of</strong> clarity related to<br />
poor method descriptions and to no indication <strong>of</strong> how frequently<br />
the <strong>bioburden</strong> test was to be conducted.<br />
Pre-sterilisation filtration<br />
Comments relating to the pre-sterilization <strong>bioburden</strong> related to<br />
aseptically filled products. In the case <strong>of</strong> the 483s, these applied to<br />
the pre-sterilization <strong>bioburden</strong> not being conducted or to the sample<br />
not being taken on the day <strong>of</strong> the filtration (the sample <strong>of</strong> the bulk<br />
product was taken the day be<strong>for</strong>e the filtration took place).<br />
A related observation was made to aseptic technique not being<br />
followed in relation to the sampling <strong>of</strong> the bulk product.<br />
Batch review<br />
The observations relating to batch review concerned the release<br />
<strong>of</strong> batches <strong>of</strong> final product where there was no clear evidence that<br />
the in-process <strong>bioburden</strong> tests had been included as part <strong>of</strong> the<br />
batch release process.<br />
Raw materials<br />
Three <strong>warning</strong> <strong>letters</strong> made reference to in-coming raw<br />
materials not being assessed <strong>for</strong> <strong>bioburden</strong> prior to being released<br />
<strong>for</strong> processing. These observations probably related to the tests <strong>for</strong><br />
<strong>microbial</strong> enumeration described in USP .<br />
Environmental connection<br />
Two <strong>warning</strong> <strong>letters</strong> made reference to high <strong>bioburden</strong> results<br />
not being linked, in the course <strong>of</strong> batch assessment or investigation,<br />
to the microorganisms commonly found in the manufacturing<br />
environment. The inference here was that the contamination<br />
to which the <strong>bioburden</strong> related to may have arisen from crosscontamination<br />
from the process area environment.<br />
In-process monitoring<br />
Observations relating to in-process monitoring related to stages<br />
<strong>of</strong> the manufacturing process where samples <strong>for</strong> <strong>bioburden</strong> were<br />
not taken, which the <strong>FDA</strong> inspector considered should be sampled.<br />
A process flow or risk assessment had not been conducted.<br />
Process hold times<br />
The 483 relating to process hold times related to a process<br />
where the intermediate product was held <strong>for</strong> a period <strong>of</strong> time prior to<br />
the next stage <strong>of</strong> processing. The <strong>FDA</strong> inspector made a comment<br />
that the hold time should be assessed in order to determine if the<br />
<strong>bioburden</strong> increased during the hold time period. Such a test would<br />
have involved sampling the product prior to the start <strong>of</strong> the hold<br />
time and at the end <strong>of</strong> the hold time.<br />
Microbial identification<br />
There were two 483 observations which discussed the<br />
characterization <strong>of</strong> microorganisms. In both cases in-process<br />
<strong>bioburden</strong> counts had exceeded the alert level and the counts had<br />
not been sent <strong>for</strong> <strong>microbial</strong> identification. This was considered to<br />
be a lack <strong>of</strong> process control.<br />
Process cleanliness<br />
There was one 483 which made specific reference to an<br />
observed lack <strong>of</strong> process hygiene and that this had not been<br />
adequately captured through testing samples <strong>for</strong> <strong>bioburden</strong>.<br />
Training<br />
There was one 483 observation which related to the person<br />
tasked with taking the sample <strong>for</strong> <strong>bioburden</strong> testing not having been<br />
trained in sampling procedures.<br />
Pharma Times - Vol. 44 - No. 12 - December 2012 30
Minutes <strong>of</strong> CEC Meeting<br />
Minutes <strong>of</strong> the CEC meeting held on 18 th August 2012 at IPA Conference<br />
room Mumbai at 2.30 pm<br />
Members Present<br />
Office Bearers<br />
Dr. J.A.S. Giri, President, Dr. C. Gopalakrishna Murty, Immediate<br />
Past President, Mrs. Manjiri Gharat, Vice President, Chairman IPA-<br />
CPD, Dr. R.N. Gupta, Vice President, Chairman IPA - HPD, Mr. S.<br />
D. Joag, Hon. Gen. Secretary, Mr. S.R. Vaidya, Hon. Treasurer,<br />
Dr. Rao V.S.V. Vadlamudi, Editor – I.J.P.S., Dr. Alka Mukne, Editor<br />
- Pharma Times<br />
Associate Secretary<br />
Dr. S.C. Mandal, Mr. Nitin Maniar<br />
Council Members<br />
Dr. Hemant Mondkar, Dr. Anand Shedge<br />
Permanent Invitees<br />
Dr. Ajit Dangi, Mr. Kaushik Desai, Mr. Subodh Priolkar, Mr. P.D.<br />
Sheth<br />
Special Invitees<br />
Pr<strong>of</strong>. V.B. Desai, Mr. Ranga Iyer, Dr. A. K. Kapadia, Mr. A.I. Mehta,<br />
Dr. S.P. Manek, Mr. S.M. Mudda<br />
Secretariat<br />
Mr. T. B. Nair, Executive Secretary<br />
Leave <strong>of</strong> Absence<br />
Dr. Tulsi Chakrabarti, Dr. Divakar Goli, Dr. Sunil K. Jain, Pr<strong>of</strong>. T.V.<br />
Narayana, Mr. Devinder Pal, Dr. M.K. Raina, Dr. T.K. Ravi, Dr.<br />
Suresh Sarvadekar, Dr. Premnath Shenoy, Dr. B. Suresh, Mr. B.N.<br />
Thakore, Mr. Raj Vaidya,<br />
1) Welcome address by the Hon. Gen. Secretary<br />
i. The Hon. Gen. Secretary welcomed all CEC members and<br />
thanked them <strong>for</strong> attending this meeting. He in<strong>for</strong>med the<br />
members about the sad demise <strong>of</strong> Mr. Ton Hoek, CEO <strong>of</strong> FIP<br />
on 20 th July 2012. Condolence message has been sent to his<br />
family and FIP Secretariat. Dr. S.C. Mandal also in<strong>for</strong>med about<br />
the sad demise <strong>of</strong> Mr. Dipankar Dutta on 7 th August 2012, who<br />
was a Life member <strong>of</strong> IPA and served IPA Bengal State branch<br />
in various positions <strong>for</strong> almost three decades. The members<br />
observed two minutes silence, as a mark <strong>of</strong> respect to the<br />
departed souls.<br />
ii. The Hon. Gen. Secretary stated that the IPA Building project<br />
will be taken up on priority and it would be completed in next<br />
two years.<br />
iii. EDQM Conference - The EDQM Conference will be held on<br />
September 28 – 29, 2012 at S<strong>of</strong>itel Hotel, Bandra Kurla Complex.<br />
The e. brochure was ready and two or more speakers from Dr.<br />
Reddy’s and Ranbaxy still had to be identified. The President<br />
requested the Vice Presidents to work towards the success <strong>of</strong><br />
the program. Mr. Ranga Iyer advised that IPA EDQM WHO<br />
Conference program announcement should be inserted in<br />
IDMA Bulletin to ensure better reach and visibility.<br />
iv. The Hon. Gen. Secretary requested Dr. Rao V.S.V. Vadlamudi<br />
to give an update on the discussions during the IPA Awards<br />
Committee. Dr. Rao Vadlamudi mentioned that while it is a<br />
very good initiative to globalize the Eminent Pharmacist award,<br />
one needs to first revisit the main objective <strong>of</strong> the award, which<br />
is to recognize the achievements and services rendered by<br />
pharmacy pr<strong>of</strong>essionals from IPA plat<strong>for</strong>m. If the organization<br />
wishes to change the objective now or in near future to include<br />
recognition to global pharmacy pr<strong>of</strong>essionals <strong>of</strong> Indian origin as<br />
well, it may not be acceptable to many in IPA who are serving<br />
the pr<strong>of</strong>ession from IPA plat<strong>for</strong>m and might very well be aspiring<br />
to be a recipient <strong>of</strong> the Highest award <strong>of</strong> IPA.<br />
2) Opening remarks by the President<br />
i. The President, Dr. J.A.S. Giri welcomed all CEC members and<br />
in<strong>for</strong>med that few important decisions were taken in the Office<br />
Bearers meeting held in the morning.<br />
ii. The President emphasized on the need to boost the image <strong>of</strong><br />
IPA with the help <strong>of</strong> senior members, state and local branches<br />
Presidents, industry and regulatory bodies. He requested Mr.<br />
Subodh Priolkar, Dr. C. Gopalakrishna Murty and Mr. P.D.<br />
Sheth to give their valuable guidance <strong>for</strong> boosting IPA’s<br />
image and promised complete support from the Office<br />
Bearers in this exercise.<br />
iii. The President expressed disappointment about the fact that IPA<br />
branches have not been as active and functional as expected.<br />
The branches have been lax in submitting their statement <strong>of</strong><br />
accounts and annual reports and many <strong>of</strong> the branches have<br />
not been holding regular elections as well.<br />
The House decided that hence<strong>for</strong>th IPA pan card and 80G<br />
certificate would not be given to any <strong>of</strong> the branches and the<br />
President requested the Hon. Treasurer to en<strong>for</strong>ce the same.<br />
In cases, where the IPA pan card has been given to any <strong>of</strong> the<br />
branches earlier, audited balance sheets <strong>of</strong> these branches<br />
should be collected by the Hon. Treasurer be<strong>for</strong>e 30 th <strong>of</strong> August<br />
2012 and amalgamated with the IPA Centre balance sheet.<br />
The Hon. Gen. Secretary remarked that it was difficult to<br />
garner participation from the branch representatives even <strong>for</strong> CEC<br />
meetings. In spite <strong>of</strong> giving support in terms <strong>of</strong> travel allowance to<br />
these representatives <strong>for</strong> attending CEC meetings, there has been<br />
no positive response. Dr. Ajit Dangi inquired about the organogram<br />
<strong>of</strong> IPA; the reporting hierarchies and so on. Mr. Ranga Iyer added<br />
that if IPA Center’s Charity Commissioner’s approval is being used<br />
by the branches, then Center becomes liable <strong>for</strong> the branch actions.<br />
Mr. Subodh Priolkar remarked that in the past, two or three branches<br />
has been disqualified on grounds <strong>of</strong> non - submission <strong>of</strong> annual<br />
reports and audited account statements and/or not holding regular<br />
elections. The branches’ share <strong>of</strong> membership fees had also been<br />
withheld in the past. The Council now had to take suitable decision<br />
regarding the non active branches. Mr. Kaushik Desai pointed out<br />
that there is a communication gap between Center and Branches.<br />
We need to look at and try and understand the problems that the<br />
branches may have been facing in complying with the expected<br />
requirements. It was decided that the Office Bearers and other<br />
senior members be appointed as Mentors <strong>for</strong> the branches,<br />
in order to supervise and guide the branch functionaries and<br />
establish a good rapport with them. The state branches would<br />
be responsible <strong>for</strong> statutory report and accounts submissions<br />
from the local branches within their states. The President<br />
requested state branch Office Bearers to follow up with their<br />
respective local branches and submit report <strong>of</strong> the local branch<br />
activities to the center.<br />
Pharma Times - Vol. 44 - No. 12 - December 2012 31