05 Classification of.. - Department of Earth and Planetary Sciences
05 Classification of.. - Department of Earth and Planetary Sciences
05 Classification of.. - Department of Earth and Planetary Sciences
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
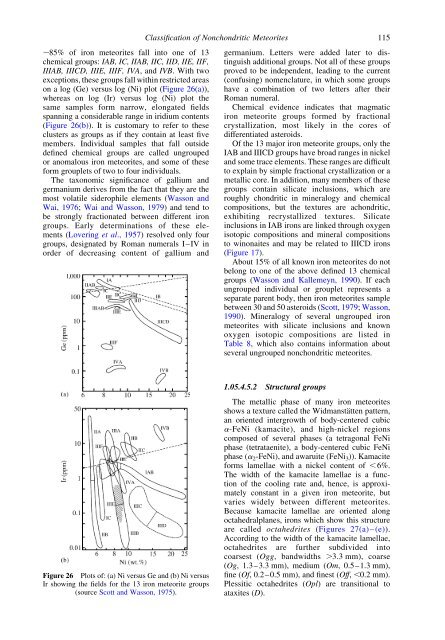
,85% <strong>of</strong> iron meteorites fall into one <strong>of</strong> 13<br />
chemical groups: IAB, IC, IIAB, IIC, IID, IIE, IIF,<br />
IIIAB, IIICD, IIIE, IIIF, IVA, <strong>and</strong> IVB. With two<br />
exceptions, these groups fall within restricted areas<br />
on a log (Ge) versus log (Ni) plot (Figure 26(a)),<br />
whereas on log (Ir) versus log (Ni) plot the<br />
same samples form narrow, elongated fields<br />
spanning a considerable range in iridium contents<br />
(Figure 26(b)). It is customary to refer to these<br />
clusters as groups as if they contain at least five<br />
members. Individual samples that fall outside<br />
defined chemical groups are called ungrouped<br />
or anomalous iron meteorites, <strong>and</strong> some <strong>of</strong> these<br />
form grouplets <strong>of</strong> two to four individuals.<br />
The taxonomic significance <strong>of</strong> gallium <strong>and</strong><br />
germanium derives from the fact that they are the<br />
most volatile siderophile elements (Wasson <strong>and</strong><br />
Wai, 1976; Wai <strong>and</strong> Wasson, 1979) <strong>and</strong> tend to<br />
be strongly fractionated between different iron<br />
groups. Early determinations <strong>of</strong> these elements<br />
(Lovering et al., 1957) resolved only four<br />
groups, designated by Roman numerals I–IV in<br />
order <strong>of</strong> decreasing content <strong>of</strong> gallium <strong>and</strong><br />
<strong>Classification</strong> <strong>of</strong> Nonchondritic Meteorites 115<br />
germanium. Letters were added later to distinguish<br />
additional groups. Not all <strong>of</strong> these groups<br />
proved to be independent, leading to the current<br />
(confusing) nomenclature, in which some groups<br />
have a combination <strong>of</strong> two letters after their<br />
Roman numeral.<br />
Chemical evidence indicates that magmatic<br />
iron meteorite groups formed by fractional<br />
crystallization, most likely in the cores <strong>of</strong><br />
differentiated asteroids.<br />
Of the 13 major iron meteorite groups, only the<br />
IAB <strong>and</strong> IIICD groups have broad ranges in nickel<br />
<strong>and</strong> some trace elements. These ranges are difficult<br />
to explain by simple fractional crystallization or a<br />
metallic core. In addition, many members <strong>of</strong> these<br />
groups contain silicate inclusions, which are<br />
roughly chondritic in mineralogy <strong>and</strong> chemical<br />
compositions, but the textures are achondritic,<br />
exhibiting recrystallized textures. Silicate<br />
inclusions in IAB irons are linked through oxygen<br />
isotopic compositions <strong>and</strong> mineral compositions<br />
to winonaites <strong>and</strong> may be related to IIICD irons<br />
(Figure 17).<br />
About 15% <strong>of</strong> all known iron meteorites do not<br />
belong to one <strong>of</strong> the above defined 13 chemical<br />
groups (Wasson <strong>and</strong> Kallemeyn, 1990). If each<br />
ungrouped individual or grouplet represents a<br />
separate parent body, then iron meteorites sample<br />
between 30 <strong>and</strong> 50 asteroids (Scott, 1979; Wasson,<br />
1990). Mineralogy <strong>of</strong> several ungrouped iron<br />
meteorites with silicate inclusions <strong>and</strong> known<br />
oxygen isotopic compositions are listed in<br />
Table 8, which also contains information about<br />
several ungrouped nonchondritic meteorites.<br />
Figure 26 Plots <strong>of</strong>: (a) Ni versus Ge <strong>and</strong> (b) Ni versus<br />
Ir showing the fields for the 13 iron meteorite groups<br />
(source Scott <strong>and</strong> Wasson, 1975).<br />
1.<strong>05</strong>.4.5.2 Structural groups<br />
The metallic phase <strong>of</strong> many iron meteorites<br />
shows a texture called the Widmanstätten pattern,<br />
an oriented intergrowth <strong>of</strong> body-centered cubic<br />
a-FeNi (kamacite), <strong>and</strong> high-nickel regions<br />
composed <strong>of</strong> several phases (a tetragonal FeNi<br />
phase (tetrataenite), a body-centered cubic FeNi<br />
phase (a 2 -FeNi), <strong>and</strong> awaruite (FeNi 3 )). Kamacite<br />
forms lamellae with a nickel content <strong>of</strong> ,6%.<br />
The width <strong>of</strong> the kamacite lamellae is a function<br />
<strong>of</strong> the cooling rate <strong>and</strong>, hence, is approximately<br />
constant in a given iron meteorite, but<br />
varies widely between different meteorites.<br />
Because kamacite lamellae are oriented along<br />
octahedralplanes, irons which show this structure<br />
are called octahedrites (Figures 27(a)–(e)).<br />
According to the width <strong>of</strong> the kamacite lamellae,<br />
octahedrites are further subdivided into<br />
coarsest (Ogg, b<strong>and</strong>widths .3.3 mm), coarse<br />
(Og, 1.3–3.3 mm), medium (Om, 0.5–1.3 mm),<br />
fine (Of, 0.2–0.5 mm), <strong>and</strong> finest (Off, ,0.2 mm).<br />
Plessitic octahedrites (Opl) are transitional to<br />
ataxites (D).