13-4 Ligands in Organometallic Chemistry
13-4 Ligands in Organometallic Chemistry
13-4 Ligands in Organometallic Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
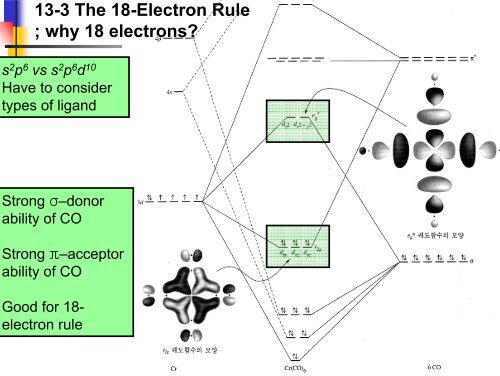
<strong>13</strong>-3 The 18-Electron Rule<br />
; why 18 electrons?<br />
s 2 p 6 vs s 2 p 6 d 10<br />
Have to consider<br />
types of ligand<br />
Strong σ–donor<br />
ability of CO<br />
Strong π–acceptor<br />
ability of CO<br />
Good for 18-<br />
electron rule