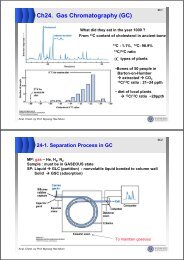
13-4 Ligands in Organometallic Chemistry
13-4 Ligands in Organometallic Chemistry
13-4 Ligands in Organometallic Chemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>13</strong>-1 Historical Background<br />
<strong>Organometallic</strong> Compound<br />
<strong>Organometallic</strong> chemistry is the study of chemical compounds<br />
conta<strong>in</strong><strong>in</strong>g bonds between carbon and a metal.<br />
<strong>Organometallic</strong> chemistry comb<strong>in</strong>es aspects of <strong>in</strong>organic<br />
chemistry and organic chemistry.<br />
<strong>Organometallic</strong> compounds f<strong>in</strong>d practical use <strong>in</strong> stoichiometric<br />
and catalytically active compounds.<br />
Electron count<strong>in</strong>g is key <strong>in</strong> understand<strong>in</strong>g organometallic<br />
chemistry. The 18-electron rule is helpful <strong>in</strong> predict<strong>in</strong>g the<br />
stabilities of organometallic compounds. <strong>Organometallic</strong><br />
compounds which have 18 electrons (filled s, p, and d orbitals)<br />
are relatively stable. This suggests the compound is isolable, but<br />
it can result <strong>in</strong> the compound be<strong>in</strong>g <strong>in</strong>ert.