World Congress of Brachytherapy 10-12 May, 2012 - Estro-events.org
World Congress of Brachytherapy 10-12 May, 2012 - Estro-events.org
World Congress of Brachytherapy 10-12 May, 2012 - Estro-events.org
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
S<strong>12</strong> <strong>World</strong> <strong>Congress</strong> <strong>of</strong> <strong>Brachytherapy</strong> 20<strong>12</strong><br />
204 patients were identified as having a high risk anatomic prostate<br />
gland (50cc, or history <strong>of</strong> prior TURP). 46 patients were<br />
identified as having ultrasound prostate volume <strong>of</strong> ≤20 cc at the time<br />
<strong>of</strong> the implant (median, 17.8cc, range 6.7cc20cc; lower quartile,<br />
6.7cc15.5cc). <strong>12</strong>1 patients were identified as having ultrasound<br />
prostate volume <strong>of</strong> ≥50 cc at the time <strong>of</strong> the implant (median, 61cc,<br />
range 50cc116cc; upper quartile 74cc116cc). 37 patients were<br />
identified as having a history <strong>of</strong> a prior TURP. In the standard risk<br />
group there were 443 patients, the median ultrasound prostate<br />
volume was 32.8cc (range, 20.249.8).<br />
Neoadjuvant hormone therapy was not routinely recommended unless<br />
the initial ultrasound prostate volume suggested significant pubic arch<br />
interference or the patient's Gleason score and or prostate specific<br />
antigen placed the patient in either intermediate or high risk<br />
categories. Patients received HDR brachytherapy as a boost either<br />
before or after 4500 cGy <strong>of</strong> external beam radiotherapy (3D conformal<br />
until 2006 and IMRT thereafter). Boost brachytherapy doses ranged<br />
from 1600 1900 cGy, given in 23 fractions, with the most common<br />
fractionation schedule being 950 cGy in two daily fractions given on<br />
the same day as the implant with a minimum 6 hours interval between<br />
fractions.<br />
: The dosimetric findings are as follows:<br />
Patient<br />
Group<br />
D90 V<strong>10</strong>0 V150 D5 rectum D5 urethra<br />
50cc Median<strong>10</strong>6.2% 95.1% 18.2% 55.4% <strong>10</strong>9.7%<br />
N=<strong>12</strong>1 Range 95117% 9099% 3.335% 3771% <strong>10</strong>3<strong>12</strong>1%<br />
TURP Median <strong>10</strong>7.8% 94.7% 19.3% 54.2% <strong>10</strong>7.8%<br />
N=37 Range <strong>10</strong>2.5117% 87.9<strong>10</strong>0% 5.332% 3474% <strong>10</strong>1<strong>12</strong>1%<br />
Standard Median <strong>10</strong>7.5% 95.5% 18% 51% 1<strong>10</strong>%<br />
N=443 Range 94117% 83<strong>10</strong>0% 336.5% 2473% 97<strong>12</strong>2%<br />
*High risk groups in bold<br />
With a median <strong>of</strong> 5.1 years, there have been nine grade II urethral<br />
strictures (CTCAE v.3) for the entire cohort. Four <strong>of</strong> these occurred in<br />
the anatomic high risk group and five occurred in the standard risk<br />
group. There was one grade II incontinence in the high risk group and<br />
one grade I incontinence in the standard risk group. There was only<br />
one grade II or higher toxicities found in the high risk group as well as<br />
only a single grade II gastrointestinal toxicity in the standard group.<br />
: In our experience, cases traditional deemed as<br />
unsuitable for prostate brachytherapy (50cc, prior TURP) are<br />
well managed with HDR brachytherapy with the expectation <strong>of</strong><br />
excellent dosimetric coverage acceptable long term toxicities.<br />
OC29<br />
SALVAGE HDR BRACHYTHERAPY FOR RECURRENT PROSTATE CANCER<br />
AFTER PRIOR DEFINITIVE RADIOTHERAPY: FIVE YEAR OUTCOMES<br />
C.P. Chen 1 , V. Weinberg 2 , K. Shinohara 3 , M. Nash 1 , A. Gottschalk 1 , M.<br />
Roach III 1 , I. Hsu 1<br />
1<br />
UCSF Comprehensive Cancer Center, Radiation Oncology, San<br />
Francisco, USA<br />
2<br />
Helen Diller Family Comprehensive Cancer Biostatistics Core,<br />
Biostatistics, San Francisco, USA<br />
3<br />
University <strong>of</strong> California San Francisco, Urology, San Francisco, USA<br />
: Evaluate the efficacy and toxicity <strong>of</strong> salvage high<br />
dose rate brachytherapy (HDRB) for locally recurrent prostate cancer<br />
after definitive radiotherapy (RT).<br />
: From November 1998 to June 2009, we<br />
retrospectively analyzed 52 consecutively accrued patients undergoing<br />
salvage HDRB for locally recurrent prostate cancer after prior<br />
definitive RT. After pathologic confirmation <strong>of</strong> locally recurrent<br />
disease, all patients received 36 Gy in 6 fractions. Twentyfour<br />
patients received neoadjuvant hormonal therapy prior to salvage<br />
whereas no patients received adjuvant hormonal therapy.<br />
Determination <strong>of</strong> biochemical failure (bF) after salvage HDRB was<br />
based on the Phoenix definition. The 5 year probability estimates <strong>of</strong><br />
overall survival (OS) and bF were calculated using KaplanMeier<br />
method. Univariate analyses were performed using the log rank test<br />
for categorical variables and Cox’s proportional hazards model for<br />
continuous variables to identify predictors <strong>of</strong> bF. Based on the<br />
Common Terminology Criteria for Adverse Events (CTCAE version 4),<br />
acute (less than 3 months postsalvage) and late (more than 3 months)<br />
genitourinary (GU) and gastrointestinal (GI) toxicities were<br />
documented.<br />
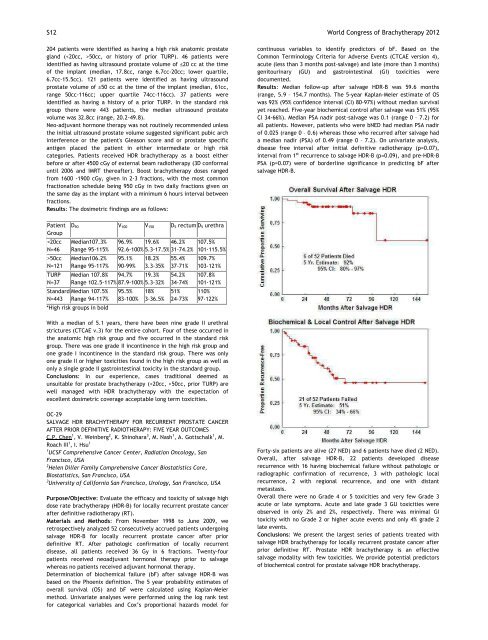
: Median followup after salvage HDRB was 59.6 months<br />
(range, 5.9 – 154.7 months). The 5year KaplanMeier estimate <strong>of</strong> OS<br />
was 92% (95% confidence interval (CI) 8097%) without median survival<br />
yet reached. Fiveyear biochemical control after salvage was 51% (95%<br />
CI 3466%). Median PSA nadir postsalvage was 0.1 (range 0 – 7.2) for<br />
all patients. However, patients who were bNED had median PSA nadir<br />
<strong>of</strong> 0.025 (range 0 – 0.6) whereas those who recurred after salvage had<br />
a median nadir (PSA) <strong>of</strong> 0.49 (range 0 – 7.2). On univariate analysis,<br />
disease free interval after initial definitive radiotherapy (p=0.07),<br />
interval from 1 st recurrence to salvage HDRB (p=0.09), and preHDRB<br />
PSA (p=0.07) were <strong>of</strong> borderline significance in predicting bF after<br />
salvage HDRB.<br />
Fortysix patients are alive (27 NED) and 6 patients have died (2 NED).<br />
Overall, after salvage HDRB, 22 patients developed disease<br />
recurrence with 16 having biochemical failure without pathologic or<br />
radiographic confirmation <strong>of</strong> recurrence, 3 with pathologic local<br />
recurrence, 2 with regional recurrence, and one with distant<br />
metastasis.<br />
Overall there were no Grade 4 or 5 toxicities and very few Grade 3<br />
acute or late symptoms. Acute and late grade 3 GU toxicities were<br />
observed in only 2% and 2%, respectively. There was minimal GI<br />
toxicity with no Grade 2 or higher acute <strong>events</strong> and only 4% grade 2<br />
late <strong>events</strong>.<br />
: We present the largest series <strong>of</strong> patients treated with<br />
salvage HDR brachytherapy for locally recurrent prostate cancer after<br />
prior definitive RT. Prostate HDR brachytherapy is an effective<br />
salvage modality with few toxicities. We provide potential predictors<br />
<strong>of</strong> biochemical control for prostate salvage HDR brachytherapy.<br />
<br />
<br />
<strong>World</strong> <strong>Congress</strong> <strong>of</strong> <strong>Brachytherapy</strong> 20<strong>12</strong> S 13<br />
<br />
<br />
<br />
OC30<br />
RESULTS OF I<strong>12</strong>5 STRANDS + EBRT +ADT IN LOCALIZED PROSTATE<br />
CANCER COMPARED TO KATTAN (2002) NOMOGRAM PREDICTIONS<br />
J. Zimmermann 1 , P. Zimmermann 2 , C. Moustakis 3<br />
1<br />
Praxiszentrum Alstertal, and Interdisziplinäres Prostatazentrum<br />
Kath. Marienkrankenhaus, Hamburg, Germany<br />
2<br />
Praxiszentrum Alstertal, Hamburg, Germany<br />
3<br />
Institut für Medizinische Physik, Klinik für Strahlentherapie,<br />
Münster, Germany<br />
: To evaluate the 5year biochemical progression<br />
free survival after prostate brachytherapy with I<strong>12</strong>5 strands +EBRT+<br />
ADT and compare the results to pretherapeutic prediction tools.<br />
Furtheron evaluation <strong>of</strong> TURP and clinical proctitis after treatment.<br />
: Between 01/2004 and <strong>12</strong>/2007, n=604<br />
consecutive patients with localized prostate carcinoma (N0M0) were<br />
treated in curative intention with iodine <strong>12</strong>5 strands (145 Gy<br />
Mono/<strong>10</strong>8 Gy with EBRT). N=71 patients had EBRT 45 Gy, n=153 ADT.<br />
Intraop preplanning in 2004 and 2005, intraop online planning in 2006<br />
and 2007. N=7 were lost to follow up, n=597 evaluable. N=313 had low<br />
risk (LR), n=204 intermediate risk (IR) and n=80 high risk (HR) disease<br />
according to the d´Amico classification. Mean follow up was 60,01<br />
months (392). The endpoint was freedom from biochemical<br />
progression (FFBP) according to the Phoenix criteria. The analysis<br />
evaluates this endpoint for risk grouping, initial PSA level, Gleason<br />
score, age, prior TURP, mean seed activity, additional ADT and more.<br />
Furtheron we looked at side effects (TURP, proctitis). The results are<br />
compared to the patient related averaged 5yearpredictions from the<br />
Kattan/MSKCC nomogram (2002) in order a. to compare the results to<br />
internationally established outcome predictions for RPX, EBRT and<br />
Brachy +EBRT (BE), b. to normalize the subgroup results and c.<br />
therefore to identify potential good or weak spots for quality<br />
assurance.<br />
: Overall FFBP was 93.3%. FFBP was 96.49% in LR group<br />
(Kattan: RPE 90%/EBRT88%/BE 89%), 90,2% in IR (Kattan: RPE<br />
75%/EBRT 77%/BE 80%) and 88,88 % in HR group (Kattan: RPE<br />
62%/EBRT 56 %/BE 81%). PSA < <strong>10</strong> ng/ml showed a FFBP <strong>of</strong> 94.35%,<br />
PSA <strong>10</strong> 20 ng/ml <strong>of</strong> 95,24 (!) %. FFBP in Gleason =8 87.5%. After TURP for<br />
LR patients, FFBP was 86.7% (Kattan: RPE 90%/EBRT 90%/BE 89%) ,<br />
after TURP for IR patients 88.89 % (Kattan: RPE 77%/EBRT 79%/BE<br />
84%) and after TURP for HR patients 75% (Kattan: RPE 62%/EBRT<br />
56%/BE 83%) . FFBP in patients < 65 years was 93.79%, 6574 years<br />
92.33% and in patients > 75 years 97.06 %. Patients without ADT had<br />
FFBP <strong>of</strong> 94.37% (Kattan: RPE 84%/EBRT82%/BE 86%), patients with ADT<br />
90.2% (Kattan: RPE 75%/EBRT74%/BE 80%).<br />
Activity per seed 0.7 mCi <strong>of</strong> 95.24%.<br />
After brachytherapy alone, TURP rate was 3,9%, rate <strong>of</strong> proctitis<br />
I/II/III was 2.1%. After brachytherapy and EBRT, TURP rate was 6.5%<br />
and proctitis I/II/III rate 8.7 %.<br />
:<br />
1. FFBP in all risk groups after a mean follow up <strong>of</strong> 60 months is<br />
excellent and compared to the patient related predictions from 2002<br />
Kattan/MSKCC nomograms signifantly better than predictions for RPX<br />
and EBRT as well as for Brachy+EBRT.<br />
2. It is possible to normalize the patients outcome in subgroups with<br />
the individual progression risks, which is especially interesting for<br />
analysis <strong>of</strong> subgroups with special aspects or individual risks.<br />
3. Thus, comparing results with the subgroup related averaged<br />
nomogram predictions might be a promising tool for intra/and<br />
interinstitutional quality assurance or clinical trials.<br />
OC31<br />
COMPARISON OF DISTANT METASTASES FOR PROSTATE CANCER<br />
PATIENTS TREATED WITH PROSTATECTOMY, BRACHYTHERAPY OR IMRT<br />
M. Zelefsky 1 , J. Eastham 2 , P. Scardino 2 , X. Pei 1 , M. Kollmeier 1 , B.<br />
Cox 1 , Y. Yamada 1<br />
1<br />
Memorial SloanKettering Cancer Center, Radiation Oncology, New<br />
York, USA<br />
2<br />
Memorial SloanKettering Cancer Center, Urology, New York, USA<br />
: To compare the longterm distant metastases<br />
free survival outcomes (DMFS) for patients with clinically localized<br />
prostate cancer treated with radical prostatectomy (RP),<br />
brachytherapy (BRT) or intensity modulated external beam<br />
radiotherapy (IMRT).<br />
: Between 1993 and 2009, 5316 patients with<br />
clinical stages T1cT2c were treated with RP (n=2870), BRT (n=1344)<br />
or IMRT (n=1<strong>10</strong>2). In the BRT cohort, 9<strong>10</strong> patients were treated with<br />
monotherapy, and 434 patients with higherrisk disease were treated<br />
with a combined modality approach which utilized supplemental IMRT<br />
to a dose <strong>of</strong> 4550.4 Gy. Patients treated with IMRT alone were<br />
treated to > 81 Gy. Patients were classified according to the NCCN<br />
risk group classification. The median follow up was 4.8 years (range:<br />
217 years).<br />
: The 7year DMFS for the RP, BRT and IMRT groups were 1.5%,<br />
0.8% and 3.6 % (p< 0.001). In the low risk group no significant<br />
differences were observed between the treatment cohorts ; the 7<br />
year DMFS rates were 0.7%, 0% and 1.2% for RP, BRT and IMRT groups<br />
(overall p value=1.0). For the intermediate risk cohort, IMRT patients<br />
experienced a higher incidence <strong>of</strong> distant metastases compared to the<br />
other cohorts; the 7year DMFS rates were 2.5%, 2.5% and 4.9% for RP,<br />
BRT and IMRT groups (p= 0.02). Cox regression analysis revealed the<br />
following variables to be associated with an inferior DMFS: higher<br />
clinical stage (p=0.004), biopsy Gleason score 7 vs 6 (p