Igneous Rocks, Composition , Texture .and Minerals
Igneous Rocks, Composition , Texture .and Minerals
Igneous Rocks, Composition , Texture .and Minerals
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Igneous</strong> <strong>Rocks</strong>, <strong>Composition</strong> , <strong>Texture</strong> .<strong>and</strong> <strong>Minerals</strong><br />
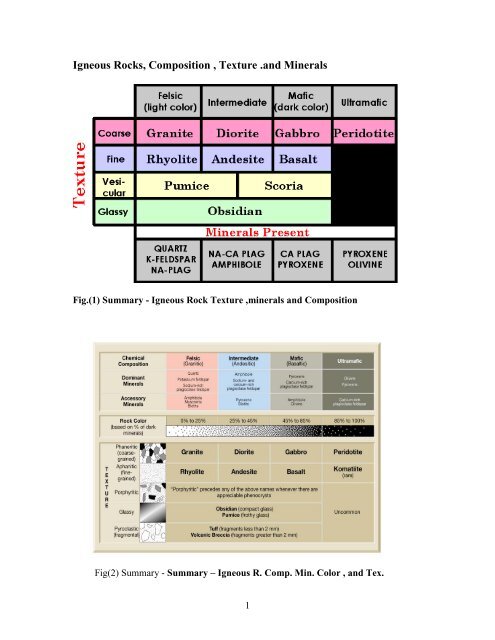
Fig.(1) Summary - <strong>Igneous</strong> Rock <strong>Texture</strong> ,minerals <strong>and</strong> <strong>Composition</strong><br />
Fig(2) Summary - Summary – <strong>Igneous</strong> R. Comp. Min. Color , <strong>and</strong> Tex.<br />
1
Fig(3) Summary - <strong>Igneous</strong> Rock , <strong>Composition</strong> , <strong>Minerals</strong> <strong>and</strong> Temperature melting<br />
Crystallization Behavior of Melts<br />
• temperatures (<strong>and</strong> pressures)<br />
• 2. Several minerals crystallize over this T range, <strong>and</strong> the number<br />
of minerals increases as T decreases<br />
• 3. The minerals that form do so sequentially, with consideral<br />
overlap<br />
• 4. <strong>Minerals</strong> that involve solid solution change composition as<br />
cooling progresses<br />
• 5. The melt composition also changes during crystallization<br />
• 6. The minerals that crystallize (as well as the sequence) depend<br />
on T <strong>and</strong> X of the melt<br />
• 7. Pressure can affect the types of minerals that form <strong>and</strong> the<br />
sequence<br />
• 8. The nature <strong>and</strong> pressure of the volatiles can also affect the<br />
minerals <strong>and</strong> their sequence<br />
2
The Phase Rule<br />
P + F = C + 2 or F = C - p + 2<br />
F = degrees of freedom<br />
The number of intensive parameters that must be specified in order to<br />
completely determine the system<br />
p = of phases<br />
phases are mechanically separable constituents<br />
C = minimum of components (chemical constituents that must<br />
be specified in order to define all phases)<br />
2 = 2 intensive parameters<br />
Usually = temperature <strong>and</strong> pressure for us geologists<br />
1 - C Systems<br />
1. The system SiO2<br />
Fig.(4): One component system SiO2, After Swamy <strong>and</strong> Saxena (1994),<br />
3
1 - C Systems<br />
The system H 2 O<br />
Fig. (5)The one component system H2O<br />
2 - C Systems<br />
A. Systems with Complete Solid Solution<br />
Fig.(6) Plagioclase (Ab-An, NaAlSi3O8 - CaAl2Si2O8)<br />
4
2. The Olivine System<br />
Fig.(7) Fo - Fa (Mg2SiO4 - Fe2SiO4) , also a solid-solution series<br />
2-C Eutectic Systems<br />
Fig.(8) Example: Diopside - AnorthiteNo solid solution<br />
5
C. Binary Peritectic Systems<br />
Three phases enstatite = forsterite + SiO2<br />
Fig(9)Three phases enstatite = forsterite + SiO2<br />
6