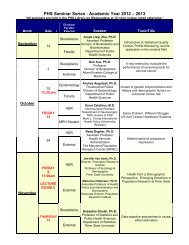
Immunofluorescence
Immunofluorescence
Immunofluorescence
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Immunofluorescent Staining Protocol <br />
Plating <br />
1. Clean desired number of coverslips (Corning 22x22mm Square #1.5) with 100% <br />
ethanol <br />
2. Place individual coverslips in the wells of a 6-‐well plate <br />
3. Add cells and incubate overnight <br />
Fixation <br />
1. Wash cells with 1X PBS quickly to remove medium <br />
2. Fix cells using 3.7% paraformaldehyde (make fresh each time) <br />
3. Incubate in paraformaldehyde for 10 minutes at room temperature <br />
4. Wash cells with 3 quick rinses of 1X PBS to remove paraformaldehyde <br />
Permeabilization <br />
1. Incubate cells in PBS + 0.2% Triton X-‐100 for 2 minutes at room temperature <br />
(punches holes in the membrane to allow antibodies to permeate the cell) <br />
2. Wash cells with 3 quick rinses of 1X PBS to remove detergent <br />
Blocking <br />
1. Incubate permeabilized cells with 5% goat serum for 30 minutes at room <br />
temperature (helps to prevent non-‐specific intracellular antibody binding – <br />
background staining) <br />
Staining <br />
*If possible, mix primary antibodies into 5% goat serum, this will also help to cut down <br />
on background staining <br />
*Concentrations for antibodies will vary, see manufacturers suggestions or previous lab <br />
protocols to determine which concentration works best <br />
1. Incubate cells in 1X PBS* + primary antibody for 1hr at room temperature <br />
2. Wash cells 4 times with PBS at room temperature (10 minutes each wash) <br />
3. Incubate cells in 1X PBS + secondary antibody for 1hr at room temperature IN THE <br />
DARK to prevent photo-‐bleaching from occurring (when using fluorescent <br />
molecules, be sure to limit the time spent in direct light to a minimum) <br />
4. Wash cells 4 times with PBS at room temperature (10 minutes each wash) – if <br />
staining with DAPI add to the first wash (have DAPI at 11mM, dilute to 300uM – use <br />
at 1:1000 dilution) <br />
Mounting <br />
1. Place a drop of mounting medium onto the slide and carefully overlay glass <br />
coverslip, CELL SIDE DOWN, onto the medium (start by making contact between <br />
the medium and coverslip at one end of the coverslip and gently lay the coverslip <br />
down onto the medium – this helps to keep the bubble formation to a minimum) <br />
2. Allow slides to dry overnight (2-‐3 hours at a minimum) IN THE DARK at room <br />
temperature <br />
3. Visualize by fluorescence microscopy
3.7% Paraformaldehyde <br />
dilute 37% formaldehyde 1:10 into PBS (needs to be made fresh each time) <br />
PBS + 0.2% Triton X-‐100 <br />
2X PHEM Buffer (500mL) <br />
18.14g PIPES <br />
6.5g HEPES <br />
3.8g EGTA <br />
0.99g MgSO4 <br />
pH to 7.0 with 10M KOH <br />
filter and store at 4°C <br />
20% Goat Serum (Heat-‐Inactivated) <br />
1. Heat desired volume of goat serum needed to make serum solution at 60-‐70°C <br />
for 1hr <br />
2. Allow serum to cool <br />
3. Add appropriate volume of 2X PHEM buffer and sodium azide to 0.05% <br />
4. Filter through 0.22uM filter and store at 4°C <br />
5. Dilute for blocking