PhD thesis
PhD thesis
PhD thesis
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Comparative neurogenesis, muscle<br />
development, and gene expression<br />
analyses in Brachiopoda<br />
<strong>PhD</strong> <strong>thesis</strong><br />
Andreas Altenburger
THE PHD SCHOOL OF SCIENCE<br />
FACULTY OF SCIENCE<br />
DEPARTMENT OF BIOLOGY<br />
UNIVERSITY OF COPENHAGEN<br />
DENMARK<br />
<strong>PhD</strong> <strong>thesis</strong><br />
Andreas Altenburger<br />
Comparative neurogenesis, muscle<br />
development, and gene expression analyses in<br />
Brachiopoda<br />
Principal supervisor<br />
Associate Prof. Dr. Andreas Wanninger<br />
Co-supervisor<br />
Prof. Dr. Pedro Martinez, University of Barcelona<br />
December , 2010
2 <br />
Principal supervisor<br />
Assoc. Prof. Dr. Andreas Wanninger<br />
Department of Biology<br />
Research Group for Comparative Zoology<br />
University of Copenhagen<br />
Copenhagen, Denmark<br />
Co-supervisor<br />
Prof. Dr. Pedro Martinez<br />
Department of Genetics<br />
University of Barcelona<br />
Barcelona, Spain<br />
Opponents<br />
Prof. Dr. Billie Swalla<br />
Department of Biology<br />
University of Washington<br />
Seattle, USA<br />
Prof. Dr. Bernard Degnan<br />
School of Biological Sciences<br />
The University of Queensland<br />
Brisbane, Australia<br />
Faculty opponent<br />
Assoc. Prof. Dr. Jørgen Olesen<br />
Zoological Museum<br />
Natural History Museum of Denmark<br />
Copenhagen, Denmark<br />
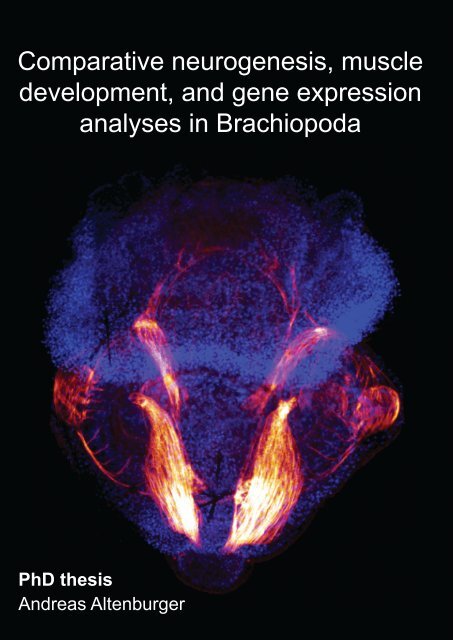
Cover legend<br />
Front: Myoanatomy of Joania (Argyrotheca) cordata. Maximum projection<br />
micrograph of a confocal laserscanning microscope stack. F-actin is labelled<br />
in red, cell nuclei are labelled in blue to indicate the outline of the specimen.<br />
Anterior faces upward and the specimen is approximately 280 µm long.<br />
Back: Schematic illustration of the specimen shown on front. The musculature<br />
comprises pedicle muscles (beige), longitudinal muscles (orange), central<br />
mantle muscles (brown), a U-shaped muscle (green), setae pouch muscles<br />
(red circles), circular mantle muscle (light blue), serial mantle muscles (dark<br />
orange), setae muscles (purple), apical longitudinal muscles (dark blue), and<br />
an apical transversal muscle (yellow).
<br />
3<br />
Content<br />
Preface ......................................................................................... 4<br />
Danish abstract ............................................................................... 5<br />
Abstract ......................................................................................... 6<br />
Short abstract ................................................................................. 7<br />
Acknowledgements ......................................................................... 8<br />
Chapter I ....................................................................................... 9<br />
Introduction ................................................................................ 9<br />
Brachiopoda .......................................................................... 9<br />
Nervous system .................................................................... 10<br />
Muscular system ................................................................... 10<br />
Gene expression ....................................................................11<br />
Material and methods ................................................................. 12<br />
Immunocytochemistry and phalloidin labeling .............................. 12<br />
Labeling of Pax3/7 proteins ..................................................... 12<br />
Detection of proliferating cells with BrdU (5-bromo-2-deoxyuridine)<br />
staining ............................................................................... 13<br />
Gene expression analyses ...................................................... 13<br />
Illustrations .......................................................................... 14<br />
Results and discussion ................................................................ 16<br />
Larval development ............................................................... 16<br />
Myogenesis ......................................................................... 20<br />
Neurogenesis with special focus on the apical organ of lophotrochozoan<br />
larvae ................................................................................. 20<br />
Distribution of Pax3/7 proteins in larvae of Terebratalia transversa 22<br />
Growth patterns of Terebratalia transversa ................................. 24<br />
Not and Cdx expression analyses ............................................. 26<br />
References ............................................................................... 28<br />
Chapter II ..................................................................................... 37<br />
Altenburger, A. & Wanninger, A. 2009 Comparative larval myogenesis<br />
and adult myoanatomy of the rhynchonelliform (articulate) brachiopods<br />
Argyrotheca cordata, A. cistellula, and Terebratalia transversa. Frontiers<br />
in Zoology 6: 1-14 ................................................................. 37<br />
Chapter III .................................................................................... 52<br />
Altenburger, A. & Wanninger, A. 2010 Neuromuscular development<br />
in Novocrania anomala: evidence for the presence of serotonin and a<br />
spiralian-like apical organ in lecithotrophic brachiopod larvae. Evolution<br />
& Development 12: 16-24 ....................................................... 52<br />
Chapter IV ................................................................................... 62<br />
Altenburger, A., Martinez, P. & Wanninger, A. First expression study of<br />
homeobox genes in Brachiopoda: the role of Not and Cdx in bodyplan<br />
patterning and germ layer specification. Submitted ...................... 62
4 <br />
Preface<br />
This <strong>thesis</strong> presents the results of three years of research at the University of<br />
Copenhagen from May 2007 until December 2010, including a research visit of<br />
one year at the University of Barcelona in 2009. The research on neurogenesis,<br />
myogenesis, and gene expression patterns in Brachiopoda was supervised by<br />
Assoc. Prof. Dr. Andreas Wanninger at the Research Group for Comparative<br />
Zoology, Department of Biology, University of Copenhagen, Denmark. The<br />
research on gene expression patterns was mainly carried out in the lab of Prof.<br />
Dr. Pedro Martinez, Department of Genetics, University of Barcelona, Spain.<br />
The <strong>PhD</strong> project was funded by The Danish Agency for Science, Technology<br />
and Innovation (grant no. 645-06-0294 to Andreas Wanninger).<br />
This project included several research visits of altogether nine weeks at the<br />
Sven Lovén Center for Marine Sciences in Kristineberg, Sweden, three weeks<br />
at the Moreton Bay Research Station on North Stradbroke Island, Australia,<br />
three weeks at the Banyuls-sur-mer Oceanological Observatory, France, and<br />
ten weeks at the Friday Harbor Laboratories, USA. Additional impact on my<br />
thinking about the field of evolution and development had the summer school on<br />
Evolution and Development of the Metazoans by Prof. Dr. Billie Swalla and Prof.<br />
Dr. Ken Halanych at the Friday Harbor Laboratories, University of Washington,<br />
USA, the Summer School on Evolutionary Developmental Biology by Prof. Dr.<br />
Alessandro Minelli and Assist. Prof. Giuseppe Fusco, University of Padua, Italy,<br />
and the EMBO course on Marine Animal Models in Evolution and Development<br />
organized by Prof. Dr. Detlev Arendt at the University of Gothenburg, Sweden.<br />
This <strong>thesis</strong> is composed of four chapters. Chapter I constitutes a short<br />
introduction to the research field and discusses the presented results in a<br />
broader perspective. Chapters II-IV contain two published papers and one<br />
submitted manuscript, which report the major findings made during this <strong>PhD</strong><br />
project.<br />
Copenhagen, December 2010<br />
Andreas Altenburger
<br />
5<br />
Danish abstract<br />
Brachiopoda udgør en dyrerække med en unik kropsbygning. Rækken omfatter<br />
ca. 370 nulevende arter opdelt i tre undergrupper, Rhynchonelliformea,<br />
Craniiformea og Linguliformea, men der er over 12.000 beskrevne fossile arter<br />
daterende helt tilbage til tidlig Kambrium. Der er uenighed om brachiopodernes<br />
fylogenetiske position som ofte debateres. Mit projekt har belyst dette problem<br />
gennem ny indsigt i brachipodernes ontogeni. Jeg har beskrevet udviklingen<br />
af nerve- og muskelsystemerne hos de rhynchonelliforme og craniiforme<br />
brachiopod larver af henholdsvis Terebratalia transversa og Novocrania<br />
anomala ved hjælp af immunohistokemiske indfarvninger kombineret med<br />
konfokal laserskanning mikroskopi og 3D-rekonstruktioner. Muskeldannelsen<br />
er beskrevet for både larver og voksne af Joania (Argyrotheca) cordata og<br />
Argyrotheca cistellula og ekspressionsmønstret af transskriptionsfaktorerne<br />
DP311, DP312 (Pax3/7) er beskrevet for larver og voksne af Terebratalia<br />
transversa. Ekspressionsmønstret af homeobox-generne TtrNot og TtrCdx er<br />
beskrevet for larver og juvenile af Terebratalia transversa ved hjælp af whole<br />
mount in situ hybridisering. De væsentligste resultater er: (1) Muskelanatomien<br />
hos rhynchonelliforme brachiopodlarver udviser stor lighed trods store forskelle i<br />
larvernes ydre morfologi. (2) Rhynchonelliforme og craniiforme brachiopodlarver<br />
af henholdsvis Terebratalia transversa og Novocrania anomala udviser et<br />
serotoninholdigt nervesystem, som omfatter fire eller otte flaskeformede celler<br />
i apikalorganet. Et sådant apikalorgan med flaskeformede celler er muligvis<br />
en morfologisk apomorfi for Lophotrochozoa. (3) Ekspressionsmønstret af<br />
TtrNot genet hos larverne af Terebratalia transversa indikerer en oprindelig<br />
funktion af dette gen i forbindelse med gastrulation, ektoderm specifikation<br />
og anlæggelse af nervebaner. For TtrCdx indikerer ekspressionsmønstret en<br />
oprindelig funktion i forbindelse med gastrulation samt dannelsen af den bageste<br />
del af det ektodermale væv hos Brachiopoda. Resultaterne bliver diskuteret<br />
i et fylogenetisk perspektiv gennem sammenligninger med andre rækker<br />
indenfor Lophotrochozoa, og implikationerne for evolutionen af Brachiopoda er<br />
fremhævet.
6 <br />
Abstract<br />
Brachiopods are a small phylum with a unique body plan comprising around<br />
370 living species and over 12.000 described fossil species dating back until the<br />
Lower Cambrian. The phylogenetic position of brachiopods is under controversial<br />
discussion. This project led to new insights into the ontogeny of brachiopods,<br />
which are divided into three clades, Rhynchonelliformea, Craniiformea,<br />
and Linguliformea. By use of immunocytochemistry combined with confocal<br />
laserscanning microscopy and 3D reconstruction software I describe the<br />
development of the nervous and muscular system in the rhynchonelliform and<br />
craniiform brachiopod larvae of Terebratalia transversa and Novocrania anomala.<br />
Myogenesis is described for larvae and adults of Joania (Argyrotheca) cordata<br />
and Argyrotheca cistellula and distribution of the transcription factor proteins<br />
DP311, DP312 (Pax3/7) for larvae and juveniles of Terebratalia transversa. The<br />
expression patterns of the developmental homeobox containing genes TtrNot<br />
and TtrCdx in larvae of Terebratalia transversa are described by use of whole<br />
mount in situ hybridization. The main results are: (1) The larval myoanatomy of<br />
rhynchonelliform brachiopod larvae is very similar, despite gross morphological<br />
differences in their outer morphology. (2) The rhynchonelliform and craniiform<br />
brachiopod larvae of Terebratalia transversa and Novocrania anomala show<br />
a serotonergic nervous system comprising eight or four flask-shaped cells<br />
in the apical organ. Such an apical organ with flask-shaped cells might be a<br />
morphological apomorphy of Lophotrochozoa. (3) The expression pattern of<br />
the TtrNot gene in larvae of Terebratalia transversa suggests an ancestral<br />
role of this gene in gastrulation and ectoderm specification in Brachiopoda.<br />
The expression pattern on TtrCdx suggests an ancestral role of this gene in<br />
gastrulation and the formation of posterior ectodermal tissue in Brachiopoda.<br />
The results are discussed in a phylogenetic framework compared to other<br />
lophotrochozoan phyla and implications of the results for the evolution of<br />
Brachiopoda are pointed out.
<br />
7<br />
Short abstract<br />
This <strong>thesis</strong> deals with selected aspects of brachiopod ontogeny. By use of<br />
immunocytochemistry combined with confocal laserscanning microscopy and<br />
3D reconstruction software the development of the nervous and muscular<br />
system of rhynchonelliform and craniiform brachiopod larvae is described. The<br />
expression patterns of the developmental homeobox containing genes TtrNot<br />
and TtrCdx are described by use of whole mount in situ hybridization. The main<br />
results are: (1) The larval myoanatomy of rhynchonelliform brachiopod larvae is<br />
similar despite gross morphological differences in their outer morphology. (2) The<br />
rhynchonelliform and craniiform brachiopod larvae show a serotonergic nervous<br />
system comprising eight or four flask-shaped cells in the apical organ. An apical<br />
organ comprising flask-shaped cells might be a morphological apomorphy of<br />
Lophotrochozoa. (3) The expression pattern of the TtrNot gene in larvae of<br />
Terebratalia transversa suggests an ancestral role of this gene in gastrulation<br />
and ectoderm specification in Brachiopoda. The expression pattern on TtrCdx<br />
suggests an ancestral role of this gene in gastrulation and the formation of<br />
posterior ectodermal tissue in Brachiopoda.
8 <br />
Acknowledgements<br />
The endeavour of such a <strong>thesis</strong> is impossible without the help of many people<br />
for whose support I am very grateful. Foremost I want to thank my principle<br />
supervisor Andreas Wanninger whose office door was always open and who<br />
did a great job in motivating and directing me towards the exciting parts of this<br />
study and especially the publication of the results.<br />
I am grateful to Pedro Martinez and his lab, namely Marta Chiodin, Amandine Bery,<br />
Eduardo Moreno, and Alexander Alsen for an inspiring time in Barcelona.<br />
I thank the teachers I had during <strong>PhD</strong> courses and who had a great influence on<br />
my thinking about the field of evo-devo, especially Billie Swalla, Ken Halanych,<br />
Alessandro Minelli, and Detlev Arendt.<br />
I thank the colleagues with whom I had the pleasure to share the room, lab,<br />
office, or a beer, Henrike Semmler, Nora Brinkmann, Tim Wollesen, Alen Kristof,<br />
Ricardo Neves, Julia Merkel, Birgit Meyer, Lennie Rotvit, Louise Würtz, Jan<br />
Bielecki, Jens Høeg, Lisbeth Haukrogh, Jan Lybeck, and visiting guests at the<br />
lab in Copenhagen.<br />
A special thank you to Anders Garm who translated the abstract into Danish.<br />
Many thanks go to the staff at the marine stations where I collected animals,<br />
in particular the Friday Harbor Laboratories, the Sven Lovén Centre for Marine<br />
Sciences, the Observatoire Océanologique de Banyuls-sur-mer, and the<br />
Moreton Bay Research Station.<br />
A special thank you goes to my wife Ruth who supported my work wherever<br />
she could and who took especially during the time in Barcelona the “burden” of<br />
caring full time almost alone for our son.<br />
This study was financially supported by a grant from the Danish Agency for<br />
Science, Technology and Innovation (grant no. 645-06-0294 to Andreas<br />
Wanninger) and a travel grant from Friday Harbor Labs to the author for<br />
participation in their summer course.
Introduction<br />
9<br />
Chapter I<br />
Introduction<br />
Brachiopoda<br />
The phylogenetic relationship of Brachiopoda is intensely debated among<br />
biologists and paleontologists alike. Brachiopods were already known by Linné,<br />
and 370 extant and more than 12.000 described fossil species are known (Linné<br />
1758; Ax 2003; Logan 2007). Brachiopods were significant members of the early<br />
Cambrian marine fauna and thus are one of the few phyla which are represented<br />
throughout the 550 million years of the Phanerozoic era, which extends from<br />
the first widespread appearance of organisms with mineralized skeletons until<br />
modern times (James et al. 1992). Historically, brachiopods have been assigned<br />
to different invertebrate groups, including molluscs (Lamarck 1801; Cuvier<br />
1805), bryozoans (Huxley 1853; Hancock 1858), bryozoans and phoronids<br />
(Hatschek 1888 ‘Tentaculata’; Hyman 1959 ‘Lophophorata’), or annelids (Morse<br />
1871). The three lophophorate groups or Brachiopoda alone have subsequently<br />
sometimes been regarded as deuterostomes (Brusca and Brusca 1990; Schram<br />
1991; Eernisse et al. 1992; Nielsen 1995). Since the appearance of molecular<br />
research tools, brachiopods have commonly been accepted to be protostomes<br />
(Field et al. 1988; Lake 1990; Halanych 1995; Hejnol et al. 2009). Brachiopod<br />
internal phylogeny distinguishes three clades; the inarticulate Linguliformea<br />
and Craniiformea and the articulate Rhynchonelliformea (Williams et al. 1996).<br />
Members of Linguliformea live buried in mud and have swimming juveniles<br />
instead of a true larval stage (Yatsu 1902). Members of craniiformea live with<br />
their ventral valve attached to stones and have two-lobed lecithotrophic larvae<br />
(Rowell 1960). Members of Rhynchonelliformea have a pedicle with which they<br />
attach themselves to rocks or other hard substrates (Williams et al. 1997). Their<br />
larvae have three lobes and are lecithotrophic (Freeman 2003). Traditionally,<br />
Linguliformea and Craniiformea have been grouped together as Inarticulata,<br />
while Rhynchonelliformea have been named Articulata because their valves<br />
are connected by a hinge (James et al. 1992).<br />
Brachiopods are certainly a comparatively minor phylum when only the number<br />
of recent species is considered. Nevertheless, they are present in all of the<br />
world’s oceans within all depth zones and the approximately 12.000 fossils<br />
species represent a rich source of paleontological information (Logan 2007).
10 Introduction<br />
Nervous system<br />
Microanatomical features related to the nervous system and the musculature of<br />
brachiopod larvae are virtually unknown. The literature on the nervous system<br />
of adult brachiopods boils down to descriptions by two authors on four species,<br />
Gryphus vitreus, Novocrania anomala, Discinisca lamellosa and Lingula anatina<br />
(van Bemmelen 1883; Blochmann 1892a, 1892b). Subsequent reviews of the<br />
same data are available from several authors (Helmcke 1939; Hyman 1959;<br />
Bullock and Horridge 1965a; Williams et al. 1997). In the rhynchonelliform<br />
brachiopod Gryphus vitreus the main body of nervous tissue is found around<br />
the esophagus and nerves emanate laterally from two ganglia, one subenteric<br />
ventral of the esophagus and one supraenteric dorsal of the esophagus<br />
(Rudwick 1970). The nervous system of brachiopod larvae or juveniles is<br />
only known for the linguliform Lingula anatina and Glottidia sp. and consists<br />
of a ventral lophophore system innervating the ciliary bands and a dorsal<br />
lophophore system innervating the body musculature (Hay-Schmidt 1992,<br />
2000). In order to fill the gap of knowledge concerning the brachiopod nervous<br />
system in rhynchonelliform and craniiform brachiopods, this study investigates<br />
the larval and juvenile neuroanatomy of Novocrania anomala (Craniiformea)<br />
and Terebratalia transversa (Rhynchonelliformea).<br />
Muscular system<br />
Adult brachiopods possess two main forms of muscular tissue. These are either<br />
bundles of muscle fibers that control the movement of the valves or myoepithelia<br />
in the lophophore (Williams et al. 1997). The muscles may be smooth, cross<br />
striated, or obliquely striated (Reed and Cloney 1977). Adult rhynchonelliform<br />
brachiopods comprise a pair of adductors, a pair of diductors, and a dorsal<br />
and a ventral pair of adjustor muscles that extend between the pedicle and the<br />
valves, moving the entire shell relative to the pedicle (Richardson and Watson<br />
1975). The adult craniiform Novocrania anomala comprises a pair of posterior<br />
as well as anterior adductors, a pair of oblique internal, and a pair of oblique<br />
lateral muscles (Bulman 1939). The muscular system of brachiopods and their<br />
larvae has been described by several authors (Hancock 1858; Kowalevski 1883;<br />
Blochmann 1892b; Helmcke 1939; Rudwick 1961; Reed and Cloney 1977), but<br />
no studies are available that use the benefit of up-to-date techniques such as<br />
immunocytochemistry in combination with confocal laserscanning microscopy<br />
and 3D reconstruction software in order to visualize in detail the more cryptic<br />
muscle sets of larval and adult brachiopods. Investigation of myogenesis was<br />
carried out in the course of the present <strong>PhD</strong> study in order to obtain a clearer<br />
picture of the entire brachiopod muscular bauplan as well as the dynamics of
Introduction<br />
11<br />
muscular remodeling during metamorphosis using the following species: Joania<br />
cordata (previously Argyrotheca cordata), Argyrotheca cistellula, Novocrania<br />
anomala, and Terebratalia transversa.<br />
Gene expression<br />
Data on the molecular processes that regulate animal development have<br />
greatly expanded within recent years (Carroll 2005). The investigation of gene<br />
families that encode signaling molecules with roles in the control of cell fate<br />
specification, proliferation, movement, and segment polarity has considerably<br />
improved our understanding of metazoan ontogeny (Davidson and Levine<br />
2008). So far, only few sequences of developmental genes have been<br />
identified in brachiopods, such as members of the Wnt gene family (Holland<br />
et al. 1991) and Hox genes (de Rosa et al. 1999), but nothing has so far been<br />
published on the expression of these genes during ontogeny. This might not<br />
be too surprising, since marine animals as little accessible as brachiopods are<br />
unlikely to be favored as candidate model organisms for this kind of studies<br />
(Sommer 2009). However, since the bauplan of some brachiopods has not<br />
changed significantly since the Early Cambrian, gene expression data from this<br />
phylum are very interesting because they may shed light on gene functions in<br />
the brachiopod ancestor. This information might contribute to understand the<br />
evolution of early bilaterian animals. In this study, the expression patterns of<br />
the developmental homeobox genes Not and Cdx were investigated in larvae<br />
of the rhynchonelliform brachiopod Terebratalia transversa. This was done in<br />
order to reveal the functions of these genes in Brachiopoda and to assess their<br />
ancestral function in animal development.<br />
Not is a homeobox gene and representatives of its family play an important role<br />
during notochord formation in vertebrates (Abdelkhalek et al. 2004). Its role in<br />
invertebrate development is not well known (Martinelli and Spring 2004). Cdx<br />
is a homeobox gene that is expressed in posterior tissues of almost all phyla<br />
investigated so far (Hejnol and Martindale 2008). In addition to the posterior<br />
tissues it was found to be expressed in mesoderm, gut, brain, and the central<br />
nervous system of mice, lancelets, and annelids, as well as in the gut of<br />
Drosophila and the mesoderm of Artemia (Macdonald and Struhl 1986; Duprey<br />
et al. 1988; Brooke et al. 1998; Copf et al. 2004; Fröbius and Seaver 2006). The<br />
gene expression patterns presented in this <strong>thesis</strong> are the first of their kind for<br />
the phylum Brachiopoda.
12 Material and methods<br />
Material and methods<br />
Immunocytochemistry and phalloidin labeling<br />
A range of morphological and molecular methods were applied to representative<br />
species of two main groups of Brachiopoda: Rhynchonelliformea and<br />
Craniiformea. The musculature was investigated by use of fluorescent<br />
conjugated phalloidin. Phalloidin is a toxin found in the mushroom Amanita<br />
phalloides and it binds irreversibly to F-actin.<br />
The antibodies applied to stain the nervous system bind specifically to neurotransmitters<br />
such as serotonin (5-Hydroxytryptamine [5 HT]), neuropeptides<br />
such as FMRFamide, or tubulins such as α-tubulin.<br />
An overview of the species investigated, the methods, and the antibodies<br />
applied is given in Table 1.<br />
Labeling of Pax3/7 proteins<br />
Arthropods and annelids generate new body segments from a posterior growth<br />
zone (Anderson 1973; Meier 1984; Scholtz and Dohle 1996). It has been<br />
proposed that the situation in Brachiopoda is comparable to the segmented<br />
Annelida (Morse 1871). The larval lobes in rhynchonelliform brachiopods<br />
suggest a segmented body plan and a segmented worm like ancestor of<br />
Brachiopoda (Morse 1873). In order to investigate if the rhynchonelliform<br />
brachiopod larvae of Terebratalia transversa show remnants of segmentation<br />
from a potentially segmented ancestor, the larvae were stained with antibodies<br />
that bind specifically on proteins of the Pax3/7 gene family.<br />
The antibodies DP311 and DP312 detect domains of the Pax 3/7 and non-Pax3/7<br />
proteins in Drosophila and Schistocerca (grasshopper) embryos (Davis et al.<br />
2005). The monoclonal antibodies were raised in mouse and made available<br />
by Michalis Averof (Institute of Molecular Biology & Biotechnology, Greece).<br />
DP311 stains the following proteins in Drosophila: paired (prd), gooseberry<br />
(gsb), gooseberry-neuro (gsbn), aristaless, homeobrain, and repo. DP312<br />
stains prd, gsb, gsbn and Rx.<br />
Larvae and juveniles of Terebratalia transversa were collected and fixed as<br />
described in Chapter II. The primary antibodies were used in a concentration of<br />
1:30 and the staining was applied as described in Chapters II and III. The stained<br />
specimens were analyzed with a Leica DM RXE 6 TL fluorescence microscope<br />
equipped with a TCS SP2 AOBS laserscanning device (Leica Microsystems,<br />
Wetzlar, Germany).
Material and methods<br />
13<br />
Detection of proliferating cells with BrdU (5-bromo-2-deoxyuridine)<br />
staining<br />
BrdU labeling was carried out, in order to identify possible growth zones in<br />
rhynchonelliform brachiopod larvae. BrdU is incorporated into the DNA of<br />
proliferating cells during the S-phase of the cell cycle. Staining of BrdU thus<br />
allows for visualization of dividing cells and their progenies. Larvae of Terebratalia<br />
transversa of the following developmental stages: 6, 11, 24, 35, 48, 60, and 96<br />
hours after fertilization (hpf) were incubated in 0.1mM BrdU (Sigma-Aldrich,<br />
St. Louis, MO, USA) in seawater at 11.5ºC for 6 – 48h. In another experiment<br />
larvae were cultured in 10mM BrdU in seawater for 30 min and subsequently<br />
the larvae were cultured in BrdU free seawater (pulse-chase experiment). After<br />
the treatment with BrdU the larvae were fixed in 4% paraformaldehyde in PBS<br />
for 1 hour at room temperature and then treated for 10 min at 37ºC in 0.01mg/<br />
ml proteinase K in PBS. After that they were kept for 10 min in 0.1N HCl on<br />
ice, 1 hour at 37ºC in 2N HCl, 1 hour in PBS with three changes, and 15 min in<br />
PBT (PBS with Tween 20). Then, the larvae were incubated in 1:500 mouseanti-BrdU<br />
antibody in PBT over night at 4 ºC, washed for 1 hour in PBS with<br />
three changes, 1 hour in 1:200 diluted TRITC, and finally 1 hour in PBS with<br />
three changes. Stained larvae were mounted in glycerol and analyzed with a<br />
Leica DM RXE 6 TL fluorescence microscope equipped with a TCS SP2 AOBS<br />
laserscanning device (Leica Microsystems, Wetzlar, Germany).<br />
Gene expression analyses<br />
The expression of developmental genes was studied by whole mount in<br />
situ hybridization (WMISH). Thereby, target mRNA is visualized with a<br />
complementary RNA probe which contains DIG labelled uridine (Digoxigenin-<br />
11-uridine-5’-triphosphate). The digoxigenin is subsequently stained with a<br />
Anti-DIG-AP, fab fragments antibody that contains alkaline phosphatase (AP)<br />
which in turn is made visible by a reaction with BCIP (5-Bromo-4-chloro-3-<br />
indolyl phosphate) and NBT (nitro blue tetrazolium chloride). In this reaction<br />
BCIP is dephosphorylated by AP and dimerizes to leucoindigo. This dimer is<br />
then oxidized by NBT to an insoluable dark blue 5,5’-dibromo-4,4’ precipitate<br />
(Trinh et al. 2007). The precipitate is visible in daylight conditions and also<br />
reflects laser light which allows the use of this technique in combination with a<br />
confocal laserscanning microscope (Jekely and Arendt 2007).<br />
There are several WMISH protocols available which usually have to be adapted<br />
to the organism they are intended for. Protocols developed for several species<br />
were tested in this study, namely one for the sea urchin Strongylocentrotus
14 Material and methods<br />
purpuratus, the cnidarian Nematostella vectensis, and the polychaete Platynereis<br />
dumerilii, respectively (Arendt et al. 2001; Long and Rebagliati 2002; Martindale<br />
et al. 2004; Venuti et al. 2004). The N. vectensis protocol was found to be the<br />
best of the tested protocols for the brachiopod Terebratalia transversa and was<br />
used accordingly to investigate the expression patterns of TtrNot and TtrCdx<br />
(Chapter IV).<br />
Illustrations<br />
Illustrations were done with Photoshop CS3 and Illustrator CS3 software<br />
(Adobe, San Jose, CA, USA).
<br />
15<br />
Table 1. List of species investigated, methods applied, and antibodies used. (+) indicates positive<br />
results, (-) indicates that no clear signal could be obtained, 5HT – stains nervous tissue, ad –<br />
adult, BrdU – 5-bromo-2-deoxyuridine (stains proliferating cells), CLSM – confocal laserscanning<br />
microscopy, DAPI – (stains nucleic acids), engrailed – labels segment boundaries in Drosophila,<br />
Immunostar – producer of antibodies, juv – juvenile, Pax 3/7 – labels segment boundaries in<br />
Drosophila, Phalloidin – stains F-actin, Sigma – Sigma-Aldrich, producer of antibodies, Tubulin<br />
– stains cilia and nervous tissue, WMISH – whole mount in situ hybridization.<br />
Clade<br />
Species<br />
Stages<br />
investigated<br />
larval juv ad<br />
Method<br />
applied<br />
Antibodies<br />
applied<br />
(signal + or -)<br />
Chapter<br />
Rhynchonelliformea<br />
Joania<br />
(Argyrotheca)<br />
cordata<br />
+ - + CLSM<br />
5 HT (Sigma) (-)<br />
DAPI (+)<br />
FMRF (-)<br />
Phalloidin (+)<br />
Tubulin (+)<br />
II<br />
Rhynchonelliformea<br />
5 HT (Sigma) (-)<br />
Argyrotheca<br />
+ - + CLSM<br />
FMRF (-)<br />
II<br />
cistellula<br />
Phalloidin (+)<br />
5 HT<br />
(Immunostar) (+)<br />
BrdU (+)<br />
Cdx (+)<br />
Rhynchonelliformea<br />
Terebratalia<br />
transversa<br />
+ + -<br />
CLSM<br />
WMISH<br />
DAPI (+)<br />
Engrailed (-)<br />
FMRF (-)<br />
I, II, IV<br />
Not (+)<br />
Pax 3/7 (+)<br />
Phalloidin (+)<br />
Tubulin (+)<br />
Phalloidin (+)<br />
Craniiformea<br />
5 HT<br />
Novocrania<br />
+ + - CLSM<br />
(Immunostar) (+)<br />
III<br />
anomala<br />
Tubulin (+)<br />
FMRF (-)
16 Results and discussion<br />
Results and discussion<br />
Larval development<br />
Terebratalia transversa, a representative of Rhynchonelliformea<br />
Larval development of Terebratalia transversa and regional specification during<br />
embryogenesis has been described previously (Freeman 1993). My results<br />
are congruent with these data. The oocyte (Fig. 1A) divides approximately 2<br />
hours after fertilization (hpf) at a water temperature of 11.5 °C and two polar<br />
bodies are formed (Fig. 1B). Cleavage is radial and the first two cleavages are<br />
holoblastic (Fig. 1B, C). The early blastula is composed of rounded cells (Fig.<br />
1D) and gastrulation occurs approximately at 19 hpf (Fig. 1E). In the gastrula,<br />
the wall of the archenteron forms contact with the cells of the ectoderm, i.e.,<br />
the blastocoel virtually disappears (Fig. 1F). Later in development the gastrula<br />
elongates and the blastopore becomes slit-like elongated (Fig. 1G). The three<br />
larval lobes start to form as the embryo elongates further and an apical tuft<br />
appears, which is lost later in development (Fig 1H, I). At this stage the larvae<br />
become positively phototactic and usually swim in the upper part of the water<br />
column. At approximately 75 hpf the larvae are almost fully developed and the<br />
apical, mantle, and pedicle lobe are formed. Only the setae continue to grow<br />
at this point of development. The fully developed larvae eventually become<br />
negatively phototactic. Then, they swim towards the bottom of the culture dish<br />
and repeatedly touch the surface with their apical lobe, probably in order to test<br />
if the substrate is suitable for metamorphosis. Larvae settle and metamorphose<br />
between 120 and 300 hpf. The juveniles still retain the larval setae and the<br />
lophophore starts to form after settlement (Fig. 1J). Metamorphosis appears to<br />
be catastrophic since all tissues seem to be reformed during metamorphosis<br />
(Stricker and Reed 1985a, 1985b).
Results and discussion<br />
17<br />
A B C<br />
D<br />
0 2 3 10<br />
at<br />
E F ec G<br />
H<br />
AL<br />
AL<br />
en<br />
* *<br />
*<br />
18 24<br />
30 36<br />
I<br />
se<br />
AL<br />
ML<br />
PL<br />
J<br />
se<br />
se<br />
se<br />
Lo<br />
75 hpf Pe 360 hpm<br />
se<br />
Figure 1. Developmental stages of Terebratalia transversa at a water temperature of 11.5 °C.<br />
Numbers indicate the age in hours after fertilization (hpf) for all stages except of J where it is<br />
hours after the onset of metamorphosis (hpm). Size of all stages is around 120 µm in diameter,<br />
except for J where it is around 200 µm. Anterior is oriented upwards and cilia are omitted for<br />
clarity. (A) unfertilized oocyte (black) with an egg shell (grey). (B) Lateral view of two cell stage<br />
with two polar bodies and the egg shell (grey). (C) Apical view of a four cell stage. (D) Sagittal<br />
section through an early blastula. (E) Sagittal section through a late blastula at the onset of<br />
gastrulation. (F) Gastrula with ectoderm (ec), endoderm (en), and blastopore (asterisk). The<br />
gastrula starts to swim at this point of development. (G) Elongated late gastrula with slit-like<br />
blastopore (asterisk) and first signs of a distinguished apical lobe (AL). (H) Larva with further<br />
developed lobes, almost closed blastopore (asterisk), and apical tuft (at). (I) Fully established<br />
larva with apical lobe (AL), mantle lobe (ML), and pedicle lobe (PL). Four sets of setae bundles<br />
(se, only two visible) originate from the mantle lobe. (J) Juvenile with lophophore (Lo), and<br />
pedicle (Pe). The remaining larval setae (se) extend beyond the two valves.<br />
se<br />
Novocrania anomala, a representative of Craniiformea<br />
Development of Novocrania anomala and regional specification during<br />
embryogenesis has been described previously (Nielsen 1991; Freeman 2000).<br />
My results are congruent with these data. However, the two authors disagree<br />
about the development of the coelom and the formation of the mesoderm.<br />
According to Nielsen, the sheet of cells that invaginates during gastrulation is<br />
composed of two cell populations, endoderm and mesoderm, whereas Freeman<br />
states that the mesoderm is formed by individual cells which immigrate from the<br />
endodermal cell layer after invagination has been completed (Nielsen 1991;<br />
Freeman 2000). Nielsen describes the coelom as consisting of an anterior<br />
coelomic pouch in the apical lobe and three pairs of coelomic cavities in the
18 Results and discussion<br />
posterior lobe of the larva, whereas Freeman denies the existence of larval<br />
coelomic structures and states that the coelom develops after the larvae have<br />
undergone metamorphosis (Nielsen 1991; Freeman 2000). The methods used<br />
here do not allow a conclusive statement concerning coelom and mesoderm<br />
formation in larvae of N. anomala, there is more work needed to resolve the<br />
controversies on an ultrastructural level.<br />
Cleavage is radial and the first two divisions are holoblastic (Fig. 2B). The gastrula<br />
is first spherical and invagination takes place at the vegetal pole of the larva.<br />
The archenteron cells come to lie opposite of the ectoderm. Subsequently, the<br />
blastocoel disappears completely (Fig. 2C). Later in development the gastrula<br />
elongates and the blastopore comes to lie at the postero-ventral side of the<br />
swimming larva (Fig. 2D). The elongated gastrula subsequently differentiates<br />
into two larval lobes, an apical lobe and a posterior lobe (Fig. 2E, F). Larval<br />
development completes with the growth of three pairs of dorsal setal bundles<br />
on the posterior lobe (Fig. 2G). Prior to settlement, the larva swims along the<br />
bottom of the culture dish, probably in order to test if the substrate is suitable<br />
for settlement. In contrast to the descriptions by Nielsen (1991), the larvae do<br />
not curl before metamorphosis. Although curled larvae are found in the culture<br />
dishes, these seem to be unable to metamorphose. What causes the curling<br />
is unclear, however it can clearly be seen in the musculature of settled larvae<br />
that the remaining larval muscles are elongated and relaxed in contrast to the<br />
contracted musculature of curled larvae (Fig. 3A, B, and Chapter III).<br />
At a water temperature of 14 °C, metamorphosis takes place around six to ten<br />
days after fertilization (dpf). During metamorphosis the larva attaches to the<br />
substrate, secretes the shell, and retains its larval lobes, which are subsequently<br />
transformed and form the lophophore and other adult organs (Figs. 2H, 3B,<br />
C).
Results and discussion<br />
19<br />
A B C D<br />
0 4<br />
25 * 32<br />
E F G H<br />
AL<br />
se<br />
se<br />
se<br />
se<br />
AL<br />
PL<br />
40 72 105<br />
se<br />
AL<br />
PL<br />
se<br />
se<br />
ec<br />
en<br />
*<br />
se<br />
se<br />
se<br />
se<br />
se<br />
s<br />
AL<br />
PL<br />
ec<br />
en<br />
se<br />
se<br />
200<br />
Figure 2. Developmental stages of Novocrania anomala at a water temperature of 14 °C.<br />
Numbers indicate the age in hours after fertilization (hpf) for all stages except for H where it is<br />
hours after the onset of metamorphosis (hpm). Size of all stages is around 130 µm in diameter.<br />
Anterior is oriented upwards. Cilia have been omitted for clarity (A) Unfertilized oocyte (black)<br />
with egg shell (grey). (B) Apical view of a four cell stage with the egg shell at 4hpf. (C) Frontal<br />
view of a gastrula with blastopore (asterisk), ectoderm (ec), and endoderm (en). The gastrula<br />
starts to swim at this point of development. (D) Lateral view of an elongated gastrula with<br />
ectoderm (ec) and endoderm (en). The blastopore (asterisk) is situated on the posterior end<br />
of the gastrula. (E) Dorsal view of an elongated gastrula with almost distinct apical lobe (AL).<br />
(F) Ventral view of an early two-lobed larva with apical lobe (AL) and posterior lobe (PL). The<br />
blastopore is closed and larval setae (se) start to grow on the posterior side. (G) Dorsal view of<br />
a fully developed larva with apical lobe (AL), posterior lobe (PL), and three pairs of dorsal setae<br />
bundles (se). (H) Ventral view of a juvenile after metamorphosis. The larval apical lobe (AL) and<br />
pedicle lobe (PL) are still visible. The juvenile shell (s) is formed on the dorsal side with larval<br />
setae (se) extending from it.<br />
Figure 3. Metamorphosis of Novocrania anomala. Scale bars equal 50 µm, anterior is up. A and<br />
B are overlays of confocal maximum projections of phalloidin stainings and light micrographs.<br />
C is a light micrograph of a live specimen. (A) Ventral view of a curled larva with contracted<br />
musculature (empty arrow), apical lobe (AL), and posterior lobe (PL). (B) Musculature of a<br />
settled juvenile with remaining elongated larval musculature (empty arrowheads), juvenile<br />
anterior adductor muscles (aad), larval setae pouch muscles (arrows), larval anterior lobe (AL),<br />
posterior lobe (PL), and juvenile shell (s). (C) Dorsal view of a settled juvenile with remaining<br />
larval setae (se), shell (s), posterior lobe (PL), and apical lobe (AL) which has started to form<br />
the lophophore (Lo).
20 Results and discussion<br />
Myogenesis<br />
Results of larval myogenesis and adult myoanatomy are presented in Chapters<br />
II and III.<br />
Actin and myosin are molecules present in all metazoans including basal groups<br />
such as sponges and Trichoplax (Thiemann and Ruthmann 1989; Kanzawa et<br />
al. 1995). It has been proposed that the basal pattern of musculature in the<br />
bilaterian ancestor was a grid of outer circular and inner longitudinal musculature,<br />
the Hautmuskelschlauch (HMS), which has in some taxa been modified in<br />
combination with the evolution of hard exoskeletons (Schmidt-Rhaesa 2007a).<br />
Brachiopods have discrete bundles of muscle fibers that control the movement<br />
of the valves and the tentacles. Brachiopods have further myoepithelia which<br />
are found on the inner side of coelomic epithelia, in the parietal bands, in mantle<br />
lobes, and in the lophophore (Williams et al. 1997). Additionally, I could show<br />
that adults of the species Joania cordata, Argyrotheca cistellula, Novocrania<br />
anomala, and Terebratalia transversa contain discrete bundles of mantle<br />
retractor muscles (Chapters II, III), a character that is probably present in all<br />
brachiopods.<br />
The larval musculature is similar among the rhynchonelliform brachiopods<br />
investigated herein (Chapter II). Remnants of a HMS could not be distinguished.<br />
Accordingly, if the ancestor of Brachiopoda had a HMS, it was lost during the<br />
evolution of this phylum. Interestingly, the larval musculature of the craniiform<br />
brachiopod Novocrania anomala is very different from the musculature of<br />
the investigated rhynchonelliform brachiopod larvae (Chapter III). This hints<br />
towards an early split in the evolution of these two groups. This is confirmed by<br />
the fossil record, which estimates the split between the rhynchonelliform and<br />
craniiform clade to have taken place before the Ordovician 485 million years<br />
ago (Freeman and Lundelius 2005).<br />
Neurogenesis with special focus on the apical organ of<br />
lophotrochozoan larvae<br />
Results on neurogenesis in brachiopod larvae and juveniles are presented in<br />
Chapters III and IV.<br />
Adult rhynchonelliform brachiopods have a nervous system which is concentrated<br />
around the esophagus and comprises two ganglia, one dorsal and one ventral of<br />
the esophagus, as well as circumenteric nerves that innervate the lophophore,<br />
ventral mantle nerves, and dorsal mantle nerves (van Bemmelen 1883; Bullock<br />
and Horridge 1965a). The nervous system of adult Novocrania anomala lacks<br />
the dorsal ganglion. The circumenteric nerves emanate laterally from the ventral
Results and discussion<br />
21<br />
ganglion and form a ring around the esophagus. Additional lateral and brachial<br />
nerves emanate from the ventral ganglion (Blochmann 1892b). The nervous<br />
system of the lecithotrophic rhynchonelliform brachiopod larvae of Terebratalia<br />
transversa comprises two sets of four serotonergic flask-shaped cells in the<br />
apical organ that are connected by neurites to a larval neuropil in the apical lobe<br />
(Chapter IV). The nervous system of the lecithotrophic craniiform brachiopod<br />
larvae of Novocrania anomala comprises four centrally positioned serotonergic<br />
flask-shaped cells in the apical organ connected to two ventral nerve cords that<br />
extend ventrolaterally along the body (Chapter III). Linguliform planktotrophic<br />
brachiopod juveniles of Lingula anatina and Glottidia sp. possess a nervous<br />
system comprising an apical ganglion as well as dorsal and ventral lophophore<br />
nerves (Hay-Schmidt 1992). The apical ganglion of Glottidia sp. contains<br />
numerous serotonergic cells that are associated with two serotonergic tracts<br />
which project into the ciliary band (Hay-Schmidt 2000). This system is probably<br />
not homologous to the apical organs found in T. transversa and N. anomala,<br />
since there are numerous serotonergic cells in Glottidia sp. and none of these<br />
cells are flask-shaped.<br />
The evolution of nervous systems has been reviewed by several authors<br />
(Bullock and Horridge 1965b; Holland 2003; Schmidt-Rhaesa 2007b; Arendt<br />
et al. 2008; Benito-Gutiérrez and Arendt 2009; Wanninger 2009; Harzsch and<br />
Wanninger 2010). All eumetazoans are able to transmit information between<br />
cells. Sponges use electric signals albeit lacking neurons (Leys et al. 1999),<br />
cnidarians have a nerve net with electrical and chemical synapses (Anderson<br />
and Trapido-Rosenthal 2009), and bilaterians have a nervous system that often<br />
comprises some sort of “brain” and nerve cords or neurite bundles (Rieger et al.<br />
2010). The last common ancestor of cnidarians and bilaterians most likely had<br />
a nerve net which developed under the control of anteroposterior patterning<br />
genes (Westfall 1996; Westfall and Elliott 2002; Watanabe et al. 2009). The<br />
question whether the ancestor of Protostomia and Deuterostomia had a diffuse<br />
nervous system or a centralized nervous system is still hotly debated and a<br />
final statement can not yet be made (Younossi-Hartenstein et al. 1997; Arendt<br />
and Nübler-Jung 1999; Holland 2003; Lowe et al. 2003; 2006; Telford 2007;<br />
De Robertis 2008; Reichert 2009; Harzsch and Wanninger 2010). Recent<br />
studies showed that larval Entoprocta and adult Mollusca show a tetraneurous<br />
condition consisting of one pair of ventral and on pair of more dorsally positioned<br />
lateral nerve cords. In addition, the creeping-type entoproct larva and the<br />
polyplacophoran larvae exhibit a complex apical organ consisting of around<br />
eight centrally positioned serotonergic flask-shaped cells which are surrounded<br />
by several peripheral cells. (Wanninger et al. 2007; Fuchs and Wanninger 2008;
22 Results and discussion<br />
Wanninger 2008; 2009). In Nemertea, the lecithotrophic, non-pilidium like larva<br />
of Quasitetrastemma stimpsoni shows a pair of serotonergic flask-shaped cells<br />
in the apical organ plus a pair of subapical cells and two posterior neurons<br />
that are located ventrolaterally (Chernyshev and Magarlamov 2010). Annelid<br />
larvae show a serotonergic apical organ comprising up to four cells. The apical<br />
organ is associated with the prototrochal nerve ring which in turn is connected<br />
to two ventral nerve cords (Voronezhskaya et al. 2003; McDougall et al. 2006;<br />
Brinkmann and Wanninger 2008). The apical organ of ectoproct cyphonautes<br />
larvae comprises two pairs of serotonergic cell bodies from which lateral nerves<br />
project towards the corona (Hay-Schmidt 2000; Gruhl 2009). One of the two cell<br />
clusters in the apical organ contains flask-shaped cells (Nielsen and Worsaae<br />
2010). In the apical organ of the ectoproct coronate larva of Bugula neritina<br />
two flask-shaped serotonergic cells are present (Pires and Woollacott 1997;<br />
Shimizu et al. 2000). In the actinotroch larva of Phoronida, the apical organ<br />
contains numerous serotonergic cells, but these are probably not flask-shaped<br />
(Santagata 2002; Santagata and Zimmer 2002; Wanninger 2008).<br />
Taken together, the data that have recently become available on lophotrochozoan<br />
larval neuroanatomy suggest that an apical organ comprising serotonergic<br />
flask-shaped cells was present in larvae of the last common lophotrochozoan<br />
ancestor (Wanninger 2008). Accordingly, an apical organ containing such cells<br />
might be a morphological apomorphy of Lophotrochozoa.<br />
Distribution of Pax3/7 proteins in larvae of Terebratalia transversa<br />
A sister group relationship of Brachiopoda with Annelida has been hypothesized<br />
based on molecular data as well as on paleontological data and is supported by<br />
the notion that annelids and brachiopods share similarities in the ultrastructure<br />
of their setae (Gustus and Cloney 1972; Orrhage 1973; Field et al. 1988; Lake<br />
1990; Conway Morris and Peel 1995; Lüter 2000b). Several developmental<br />
genes that are involved in the establishment of segments and segmentation in<br />
animals have been characterized, some of which belong to the Pax3/7 group.<br />
Pax3 and Pax7 genes probably arose by duplication from unique ancestral Pax3/7<br />
genes and have similarities in their protein sequence and expression (Hayashi et<br />
al. 2010). Pax3/7 genes are also known as Pax group III genes and include the<br />
pair-rule gene paired (prd), the segment polarity genes gooseberry (gsb), and<br />
gooseberry-neuro (gsbn), a gene that is expressed in the developing nervous<br />
system and, together with engrailed, establishes the posterior commissures in<br />
the fruit fly Drosophila melanogaster (Noll 1993; Colomb et al. 2008). Together<br />
with their vertebrate homologs (Pax-3 and Pax-7) the Pax3/7 group forms one
Results and discussion<br />
23<br />
of four classically defined subgroups of the Pax family transcription factors<br />
(Balczarek et al. 1997). Pax3/7 shares its expression among distantly related<br />
insects and shows several patterns including pair-rule, segment polarity,<br />
and neural patterning (Davis et al. 2005). In crustaceans Pax3/7 genes are<br />
expressed in iterated stripes (Davis et al. 2005). In myriapods and chelicerates<br />
Pax3/7 gene expression exhibits iterated stripes that form early in the posteriormost<br />
part of the germ band (Davis et al. 2005). In the tardigrade Hypsibius<br />
dujardini, the Pax3/7 proteins localize in a segmentally iterated pattern in the<br />
ectoderm, after establishment of endomesoderm segmentation, but before the<br />
visible segmentation of the ectoderm (Gabriel and Goldstein 2007). Pax3/7 is<br />
also localized within the developing head region of the tardigrade embryo, but<br />
no pair-rule pattern is visible during any stage of embryogenesis (Gabriel and<br />
Goldstein 2007). Tardigrades, together with arthropods and onychophorans<br />
belong to Panarthropoda (Halanych 2004).The expression pattern of Pax3/7 in<br />
H. dujardini suggests that the pair-rule function of Pax3/7 may have arisen near<br />
the base of Arthropoda.<br />
In the annelid Platynereis dumerilii Pax3/7 proteins are found in the peripheral<br />
nervous system (Kerner et al. 2009). In larvae of the brachiopod Terebratalia<br />
transversa DP311 and DP312 show identical staining patterns. Pax 3/7 starts<br />
to be present in four cells of the apical lobe in the late elongated gastrula (Fig.<br />
4B). The cells containing Pax3/7 products are later distributed in a ring on the<br />
apical lobe of early three-lobed larvae without setae (Fig. 4C). Fully established<br />
larvae show a loose distribution of cells that contain Pax3/7 products in their<br />
apical lobe (Fig. 4D, E). In juveniles Pax3/7 containing cells are mainly found<br />
in the growing lophophore (Fig. 4F). The presence of Pax3/7 gene products<br />
in the apical lobe indicates a function of those genes during neurogenesis in<br />
T. transversa. However, further experiments are necessary in order to assess<br />
whether the staining specifically shows Pax3/7 protein products, since the<br />
antibodies used were developed against the Pax3/7 sequences of Drosophila<br />
melanogaster. Ideally, cloning of the sequences of the Pax3/7 homologs of<br />
Terebratalia transversa should be carried out, followed by mapping of the<br />
epitopes of DP311 and DP312 on peptide arrays with the known peptide<br />
sequences of T. transversa and other metazoans (Harlow and Lane 1999). The<br />
final proof would then be in situ hybridizations with the specific corresponding<br />
probes. In addition, a double staining with serotonin would be necessary in<br />
order to prove that the cells containing Pax3/7 gene products are co-localized<br />
with the nervous system.
24 Results and discussion<br />
Growth patterns of Terebratalia transversa<br />
Figure 4. Staining of<br />
Pax3/7 proteins with<br />
DP311. Overlay of confocal<br />
maximum projections on<br />
light micrographs. Anterior<br />
is up and scale bars equal<br />
50 µm. (A) Gastrula with<br />
blastopore (asterisk) and<br />
no signal. (B) Late gastrula<br />
with slit-like blastopore<br />
(asterisk). Pax3/7 proteins<br />
are stained in four cells<br />
in the future apical lobe<br />
(al). (C) Early three-lobed<br />
larva with almost closed<br />
blastopore (asterisk).<br />
Pax3/7 proteins are present<br />
in several cells of the apical<br />
lobe (al) and distributed in<br />
a ring around it. No signal<br />
is found in the mantle lobe<br />
(ml) and in the pedicle lobe<br />
(pl) (D) Lateral view of a<br />
larva with apical lobe (al),<br />
mantle lobe (ml), pedicle<br />
lobe (pl), and setae (se).<br />
Pax3/7 protein containing<br />
cells are concentrated in<br />
the dorsal part of the apical<br />
lobe. (E) Fully established<br />
larva with apical lobe (al),<br />
mantle lobe (ml), pedicle<br />
lobe (pl), and setae (se).<br />
Cells with Pax3/7 proteins<br />
are loosely distributed in<br />
the apical lobe. (F) Juvenile<br />
after metamorphosis.<br />
Pax3/7 proteins are loosely<br />
expressed in the developing<br />
lophophore (Lo) of the<br />
juvenile. The dorsal shell (s)<br />
of this specimen is slightly<br />
shifted upwards relative<br />
to its natural position, and<br />
larval setae (se) extend out<br />
of the valves<br />
In order to identify possible growth zones in brachiopod larvae, proliferating<br />
cells in Terebratalia transversa were labeled with 5-bromo-2-deoxyuridine<br />
(BrdU). Dividing cells are equally distributed in the blastula stage (Fig. 5A),<br />
the gastrula (Fig. 5B), and the elongated gastrula (Fig. 5C). In the elongated<br />
gastrula, cells divide mostly in the center of the larva and form the mantle lobe,<br />
which is marked by a ring of dividing cells (Fig. 5D). Thereafter, dividing cells<br />
are again equally distributed throughout the larva (Fig. 5E). Larvae competent<br />
for metamorphosis also show an equal distribution of proliferating cells after a<br />
pulse-chase experiment, which once again indicates that there are no distinct<br />
growth zones that form most parts of the larval body, but that dividing cells are<br />
found throughout the developing specimen (Fig. 5F). The BrdU data suggest<br />
that from the viewpoint of proliferation zones, there are no similarities between
Results and discussion<br />
25<br />
Figure 5. Pattern of<br />
BrdU staining in larvae of<br />
Terebratalia transversa.<br />
Overlay of confocal<br />
maximum projections and<br />
light micrographs. Scale<br />
bars equal 50 µm. All stages<br />
show an equal distribution<br />
of proliferating cells,<br />
there are thus no distinct<br />
growth zones identifiable.<br />
(A) Blastula. (B) Early<br />
gastrula with blastopore<br />
(asterisk). (C) Late slightly<br />
elongated gastrula with<br />
blastopore (asterisk). (D)<br />
Early three lobed stage<br />
with the developing apical<br />
lobe (al), mantle lobe (ml),<br />
and pedicle lobe (pe). (E)<br />
Three lobed stage with<br />
apical lobe (al), mantle<br />
lobe (ml), and pedicle lobe<br />
(pl). This stage is at the<br />
onset of setae formation.<br />
(F) Fully developed threelobed<br />
stage with apical<br />
lobe (al), mantle lobe (ml),<br />
and pedicle lobe (pl).<br />
the development of Annelida and Brachiopoda. For annelids, it has been shown<br />
that, although the post-metamorphic segments originate from a posterior growth<br />
zone, the precise location of the growth zone can vary (Seaver et al. 2005;<br />
Brinkmann and Wanninger 2010). However, the rhynchonelliform brachiopods<br />
are regarded derived amongst brachiopod subgroups (Carlson 1995). The<br />
distribution of proliferating cells in Terebratalia transversa can therefore not<br />
completely rule out the possibility that the brachiopod ancestor had a growth<br />
zone. Similar experiments in linguliform and craniiform brachiopods are needed<br />
in order to further assess this issue.<br />
The Annelida-Brachiopoda sister group hypo<strong>thesis</strong> based on the ultrastructure<br />
of the setae has been questioned by Lüter who showed that there is a difference<br />
in the ultrastructure of larval and adult setae in the brachiopods Lingula anatina,<br />
Notosaria nigricans, and Calloria inconspicua, suggesting a convergent<br />
evolution of setae in Annelida and Brachiopoda (Lüter 2000b). An additional
26 Results and discussion<br />
argument against segmentation in brachiopod larvae is that the segmented<br />
appearance with three larval lobes is not recognizable by the inner bauplan<br />
on the ultrastructural level (Lüter 2000a). This has been shown for Notosaria<br />
nigricans and Calloria inconspicua. In these species, a single coelomic anlage<br />
forms one compartment with all mesodermally derived cells separated only by<br />
cellular membranes. Thus, there is only one mesoderm compartment in these<br />
larvae, which encloses one coelomic cavity (Lüter 2000a). In the segmented<br />
Annelida the coelom forms one pair of coelomic cavities in each segment<br />
(Anderson 1973).<br />
Not and Cdx expression analyses<br />
Results of gene expression patterns of the homeobox genes TtrNot and TtrCdx<br />
are presented in Chapter IV.<br />
In Terebratalia transversa, the ortholog of the homeobox gene Not, TtrNot, is<br />
expressed in the ectoderm from the beginning of gastrulation until completion<br />
of larval development, which is marked by a three-lobed body with larval setae.<br />
Expression starts at gastrulation in two areas lateral to the blastopore and<br />
subsequently extends over the animal pole of the gastrula. With elongation of<br />
the gastrula, expression at the animal pole narrows to a small band, whereas<br />
the areas lateral to the blastopore shift slightly towards the future anterior region<br />
of the larva. Upon formation of the three larval body lobes, TtrNot expressing<br />
cells are present only in the posterior part of the apical lobe. Expression ceases<br />
entirely at the onset of larval setae formation. TtrNot expression is absent in<br />
unfertilized eggs, in embryos prior to gastrulation, and in settled individuals<br />
during and after metamorphosis. Comparison to the expression patterns of Not<br />
genes in other metazoan phyla suggests an ancestral role in gastrulation, germ<br />
layer (ectoderm) specification, and neural patterning, with co-opted functions in<br />
notochord formation in chordates and left/right determination in ambulacrarians<br />
and vertebrates (Chapter IV).<br />
In Terebratalia transversa the ParaHox gene TtrCdx is expressed on the<br />
posterior side of the blastopore and its expression stays in this region until the<br />
three-lobed larva is fully formed. The expression of TtrCdx suggests a function<br />
of this gene during gastrulation and ectoderm patterning in Brachiopoda. The<br />
pattern of Cdx in other metazoans ranges from expression in the mesoderm,<br />
gut, brain, central nervous system to posterior tissues (Fröbius and Seaver<br />
2006). The basal function of Cdx is probably in patterning of posterior tissues.
General conclusions and perspectives for future research<br />
27<br />
General conclusions and perspectives for future research<br />
The results presented herein are the first developmental gene expression<br />
studies in Brachiopoda, as well as the first detailed comparative description of<br />
myogenesis and neurogenesis in brachiopod larvae based on antibody staining,<br />
confocal laserscanning microscopy, and 3D reconstruction software. This study<br />
shows that microanatomical data can yield new insights into the evolution and<br />
development of lesser known metazoan phyla such as Brachiopoda. It provides<br />
the first evidence of an apical organ in brachiopod larvae that comprises<br />
serotonergic flask-shaped cells, similar to those found in ectoprocts and<br />
spiralians. This result strongly suggests that such an apical organ constitutes a<br />
morphological apomorphy of Lophotrochozoa.<br />
Gene expression analyses of TtrNot imply an ancestral role of this gene in<br />
gastrulation and ectoderm specification in Brachiopoda. The function of Not in<br />
notochord formation in chordates and left/right determination in ambulacrarians<br />
and vertebrates might thus be co-opted in these deuterostome clades. Analysis<br />
of the TtrCdx gene expression suggests an ancestral role in gastrulation and the<br />
formation of posterior tissues in Brachiopoda as well as in Bilateria in general.<br />
Further studies should extend the database of brachiopod morphogenesis<br />
and gene expression patterns to more organ systems as well as to the third<br />
brachiopod subtaxon, Linguliformea. This would allow for a full representation<br />
of the phylum Brachiopoda with its three clades Craniiformea, Linguliformea,<br />
and Rhynchonelliformea and should allow significant inferences concerning<br />
gene function and organ system evolution within this lophophorate phylum.<br />
Such data would allow insights into the evolution of organ systems, and body<br />
plans in Brachiopoda. Additionally, investigation of gene expression patterns in<br />
Brachiopoda is needed in order to compare the function of genes, co-option,<br />
and ancestral gene functions among Brachiopoda and other animal phyla. An<br />
expressed sequence tags or genome-based approach would be the best choice<br />
in order to obtain the sequences of the whole range of developmental genes.<br />
Preferably, this should be done for one representative of each brachiopod clade.<br />
Morphological and molecular data together would facilitate the reconstruction of<br />
the evolution of organ systems in Brachiopoda once the phylogenetic position<br />
of Brachiopoda and its sister groups has been settled.
28 References<br />
References<br />
Abdelkhalek, H. B., Beckers, A., Schuster-Gossler, K., Pavlova, M. N.,<br />
Burkhardt, H., Lickert, H., Rossant, J., Reinhardt, R., Schalkwyk, L. C.,<br />
Müller, I., Herrmann, B. G., Ceolin, M., Rivera-Pomar, R., and Gossler, A.<br />
2004. The mouse homeobox gene Not is required for caudal notochord<br />
development and affected by the truncate mutation. Genes Dev 18:1725-<br />
1736.<br />
Anderson, D. 1973. Embryology and Phylogeny in Annelids and Arthropods.<br />
Oxford: Pergamon Press Ltd.<br />
Anderson, P. and Trapido-Rosenthal, H. 2009. Physiological and chemical<br />
analysis of neurotransmitter candidates at a fast excitatory synapse in<br />
the jellyfish Cyanea capillata (Cnidaria, Scyphozoa). Invert Neurosci<br />
9:167-173.<br />
Arendt, D., Denes, A. S., Jékely, G., and Tessmar-Raible, K. 2008. The evolution<br />
of nervous system centralization. Philos Trans R Soc Lond B Biol Sci<br />
363:1523-1528.<br />
Arendt, D. and Nübler-Jung, K. 1999. Comparison of early nerve cord<br />
development in insects and vertebrates. Development 126:2309-2325.<br />
Arendt, D., Technau, U., and Wittbrodt, J. 2001. Evolution of the bilaterian larval<br />
foregut. Nature 409:81-85.<br />
Ax, P. 2003. Multicellular animals: Order in nature - system made by man. Vol.<br />
III. Heidelberg: Springer.<br />
Balczarek, K. A., Lai, Z. C., and Kumar, S. 1997. Evolution of functional<br />
diversification of the paired box (Pax) DNA-binding domains. Mol Biol<br />
Evol 14:829-842.<br />
Benito-Gutiérrez, È. and Arendt, D. 2009. CNS evolution: New insight from the<br />
mud. Curr Biol 19:R640-R642.<br />
Blochmann, F. 1892a. Ueber die Anatomie und die verwandtschaftlichen<br />
Beziehungen der Brachiopoden. Arch. Freunde Naturgesch. Mecklenbg.<br />
46:37-50.<br />
Blochmann, F. 1892b. Untersuchungen über den Bau der Brachiopoden. Jena:<br />
Gustav Fischer.<br />
Brinkmann, N. and Wanninger, A. 2008. Larval neurogenesis in Sabellaria<br />
alveolata reveals plasticity in polychaete neural patterning. Evol Dev<br />
10:606-618.<br />
Brinkmann, N. and Wanninger, A. 2010. Integrative analysis of polychaete<br />
ontogeny: cell proliferation patterns and myogenesis in trochophore<br />
larvae of Sabellaria alveolata. Evol Dev 12:5-15.
References<br />
29<br />
Brooke, N. M., Garcia-Fernandez, J., and Holland, P. W. H. 1998. The ParaHox<br />
gene cluster is an evolutionary sister of the Hox gene cluster. Nature<br />
392:920-922.<br />
Brusca, R. C. and Brusca, G. J. 1990. Invertebrates: Sunderland, Mass: Sinauer<br />
Associates.<br />
Bullock, T. H. and Horridge, G. A. 1965a. Lophophorate phyla: Ectoprocta,<br />
Brachiopoda, and Phoronida. In Structure and function in the nervous<br />
system of invertebrates. New York: W.H. Freeman.<br />
Bullock, T. H. and Horridge, G. A. 1965b. Structure and function in the nervous<br />
system of invertebrates. New York: W.H. Freeman.<br />
Bulman, O. M. B. 1939. Muscle systems of some inarticulate brachiopods. Geol<br />
Mag 76:434-444.<br />
Carlson, S. J. 1995. Phylogenetic relationships among extant brachiopods.<br />
Cladistics 11:131-197.<br />
Carroll, S. 2005. From DNA to diversity: molecular genetics and the evolution of<br />
animal design. 2 ed. Oxford: Blackwell Publishing.<br />
Chernyshev, A. V. and Magarlamov, T. Y. 2010. The first data on the nervous<br />
system of hoplonemertean larvae (Nemertea, Hoplonemertea). Gen Biol<br />
430:48-50.<br />
Colomb, S., Joly, W., Bonneaud, N., and Maschat, F. 2008. A concerted action<br />
of engrailed and gooseberry-neuro in neuroblast 6-4 is triggering the<br />
formation of embryonic posterior commissure bundles. PLoS ONE<br />
3:e2197.<br />
Conway Morris, S. and Peel, J. S. 1995. Articulated halkieriids from the Lower<br />
Cambrian of North Greenland and their role in early protostome evolution.<br />
Philos Trans R Soc Lond B Biol Sci 347:305-358.<br />
Copf, T., Schröder, R., and Averof, M. 2004. Ancestral role of caudal genes in<br />
axis elongation and segmentation. Proc Natl Acad Sci USA 101:17711-<br />
17715.<br />
Cuvier, G. L. 1805. Leçons d’anatomie comparée de G. Cuvier. Vol. 3. Paris.<br />
Davidson, E. H. and Levine, M. S. 2008. Properties of developmental gene<br />
regulatory networks. Proc Natl Acad Sci USA 105:20063-20066.<br />
Davis, G. K., D’Alessio, J. A., and Patel, N. H. 2005. Pax3/7 genes reveal<br />
conservation and divergence in the arthropod segmentation hierarchy.<br />
Dev Biol 285:169-184.<br />
De Robertis, E. M. 2008. Evo-devo: variations on ancestral themes. Cell<br />
132:185-195.<br />
de Rosa, R., Grenier, J. K., Andreeva, T., Cook, C. E., Adoutte, A., Akam, M.,<br />
Carroll, S. B., and Balavoine, G. 1999. Hox genes in brachiopods and
30 References<br />
priapulids and protostome evolution. Nature 399:772-776.<br />
Duprey, P., Chowdhury, K., Dressler, G. R., Balling, R., Simon, D., Guenet, J.<br />
L., and Gruss, P. 1988. A mouse gene homologous to the Drosophila<br />
gene caudal is expressed in epithelial cells from the embryonic intestine.<br />
Genes Dev 2:1647-1654.<br />
Eernisse, D. J., Albert, J. S., and Anderson, F. E. 1992. Annelida and Arthropoda<br />
are not sister taxa: a phylogenetic analysis of spiralian metazoan<br />
morphology. Syst Biol 41:305-330.<br />
Field, K., Olsen, G., Lane, D., Giovannoni, S., Ghiselin, M., Raff, E., Pace, N.,<br />
and Raff, R. 1988. Molecular phylogeny of the animal kingdom. Science<br />
239:748-753.<br />
Freeman, G. 1993. Regional specification during embryogenesis in the articulate<br />
brachiopod Terebratalia. Dev Biol 160:196-213.<br />
Freeman, G. 2000. Regional specification during embryogenesis in the craniiform<br />
brachiopod Crania anomala. Dev Biol 227:219-238.<br />
Freeman, G. 2003. Regional specification during embryogenesis in<br />
Rhynchonelliform brachiopods. Dev Biol 261:268-287.<br />
Freeman, G. and Lundelius, J. W. 2005. The transition from planktotrophy to<br />
lecithotrophy in larvae of Lower Palaeozoic rhynchonelliform brachiopods.<br />
Lethaia 38:219-254.<br />
Fröbius, A. and Seaver, E. 2006. ParaHox gene expression in the polychaete<br />
annelid Capitella sp. I. Dev Genes Evol 216:81-88.<br />
Fuchs, J. and Wanninger, A. 2008. Reconstruction of the neuromuscular system<br />
of the swimming-type larva of Loxosomella atkinsae (Entoprocta) as<br />
inferred by fluorescence labelling and confocal microscopy. Org Divers<br />
Evol 8:325-335.<br />
Gabriel, W. and Goldstein, B. 2007. Segmental expression of Pax3/7 and<br />
Engrailed homologs in tardigrade development. Dev Genes Evol<br />
217:421-433.<br />
Giribet, G. 2008. Assembling the lophotrochozoan (=spiralian) tree of life. Philos<br />
Trans R Soc Lond B Biol Sci 363:1513-1522.<br />
Gruhl, A. 2009. Serotonergic and FMRFamidergic nervous systems in<br />
gymnolaemate bryozoan larvae. Zoomorphology 128:135-156.<br />
Gustus, R. M. and Cloney, R. A. 1972. Ultrastructural similarities between setae<br />
of brachiopods and polychaetes. Acta Zool 53:229-233.<br />
Halanych, K. M. 1995. Evidence from 18S ribosomal DNA that the lophophorates<br />
are protostome animals. Science 268:485-485.<br />
Halanych, K. M. 2004. The new view of animal phylogeny. Annu Rev Ecol Evol<br />
Syst 35:229-256.
References<br />
31<br />
Hancock, A. 1858. On the organization of the Brachiopoda. Phil. Trans. R. Soc.<br />
Lond. 148:791-869.<br />
Harlow, E. and Lane, D. 1999. Using antibodies: A laboratory manual. Cold<br />
Spring Harbor, New York: Cold Spring Harbor Laboratory Press.<br />
Harzsch, S. and Wanninger, A. 2010. Evolution of invertebrate nervous systems:<br />
the Chaetognatha as a case study. Acta Zool 91:35-43.<br />
Hatschek, B. 1888. Lehrbuch der Zoologie: eine morphologische Übersicht<br />
des Thierreiches zur Einführung in das Studium dieser Wissenschaft:<br />
G. Fischer.<br />
Hay-Schmidt, A. 1992. Ultrastructure and immunocytochemistry of the nervoussystem<br />
of the larvae of Lingula anatina and Glottidia sp. (Brachiopoda).<br />
Zoomorphology 112:189-205.<br />
Hay-Schmidt, A. 2000. The evolution of the serotonergic nervous system. Proc<br />
R Soc B 267:1071-1079.<br />
Hayashi, S., Drayton, B., Aurade, F., Rocancourt, D., Buckingham, M., and<br />
Relaix, F. 2010. Conserved functions of Pax3/7 during evolution. Dev<br />
Biol 344:528-529.<br />
Hejnol, A. and Martindale, M. Q. 2008. Acoel development indicates the<br />
independent evolution of the bilaterian mouth and anus. Nature 456:382-<br />
386.<br />
Hejnol, A., Obst, M., Stamatakis, A., Ott, M., Rouse, G. W., Edgecombe, G. D.,<br />
Martinez, P., Baguñà, J., Bailly, X., Jondelius, U., Wiens, M., Müller, W.<br />
E. G., Seaver, E., Wheeler, W. C., Martindale, M. Q., Giribet, G., and<br />
Dunn, C. W. 2009. Assessing the root of bilaterian animals with scalable<br />
phylogenomic methods. Proc R Soc B 276:4261-4270.<br />
Helmcke, J. G. 1939. Die Muskeln der Brachiopoden. Zool Jahrb Abt Syst Oekol<br />
Geogr Tiere 72:100-140.<br />
Holland, N. D. 2003. Early central nervous system evolution: an era of skin<br />
brains? Nat Rev Neurosci 4:617-627.<br />
Holland, P. W. H., Williams, N. A., and Lanfear, J. 1991. Cloning of segment<br />
polarity gene homologues from the unsegmented brachiopod<br />
Terebratulina retusa (Linnaeus). FEBS Letters 291:211-213.<br />
Huxley, T. H. 1853. On the morphology of the cephalous mollusca, as illustrated<br />
by the anatomy of certain Heteropoda and Pteropoda collected during<br />
the voyage of H.M.S. “Rattlesnake” in 1846-50. Phil. Trans. R. Soc.<br />
Lond. 143:29-65.<br />
Hyman, L. H. 1959. The lophophore coelomates - phylum Brachiopoda. In<br />
The Invertebrates Smaller Coelomate Groups, edited by L. H. Hyman.<br />
London: McGraw-Hill Book Company.
32 References<br />
James, M. A., Ansell, A. D., Collins, M. J., Curry, G. B., Peck, L. S., and Rhodes,<br />
M. C. 1992. Biology of living brachiopods. Adv Mar Biol 28:175-387.<br />
Jekely, G. and Arendt, D. 2007. Cellular resolution expression profiling using<br />
confocal detection of NBT/BCIP precipitate by reflection microscopy.<br />
Biotechniques 42:751-755.<br />
Kanzawa, N., Takano-Ohmuro, H., and Maruyama, K. 1995. Isolation and<br />
characterization of sea sponge myosin. Zool Sci 12:765-769.<br />
Kerner, P., Béhague, J., Simionato, E., Gouar, M. L., Balavoine, G., and Vervoort,<br />
M. 2009. Molecular analysis of the architecture and the development of<br />
the peripheral nervous system of the annelid Platynereis dumerilii. Mech<br />
Dev 126:S258-S258.<br />
Kowalevski, A. O. 1883. Observations sur le développement des brachiopodes<br />
(Analyse par Oehlert et Deniker). Arch Zool Exp Gen. 2:57-76.<br />
Lake, J. A. 1990. Origin of the Metazoa. Proc Natl Acad Sci USA 87:763-766.<br />
Lamarck, J. B. 1801. Classe premiere. La 5e du règne animal. Les Mollusques.<br />
In Système des Animaux sans vertèbres, edited by Déterville. Paris.<br />
Leys, S., Mackie, G., and Meech, R. 1999. Impulse conduction in a sponge. J<br />
Exp Biol 202:1139-1150.<br />
Linné, C. v. 1758. Systema naturae per regna tria naturae: secundum classes,<br />
ordines, genera, species, cum characteribus, differentiis, synonymis,<br />
locis. Vol. 1. Holmiae: Impensis Direct. Laurentii Salvii.<br />
Logan, A. 2007. Geographic distribution of extant articulated brachiopods. In<br />
Treatise on Invertebrate Paleontology, Part H, Brachiopoda, Revised,<br />
edited by P. A. Selden. Boulder, Colorado, and Lawrence, Kansas: The<br />
Geological Society of America, Inc. and The University of Kansas.<br />
Long, S. and Rebagliati, M. 2002. Sensitive two-color whole-mount in situ<br />
hybridizations using digoxygenin- and dinitrophenol-labeled RNA probes.<br />
Biotechniques 32:494-500.<br />
Lowe, C. J., Terasaki, M., Wu, M., Freeman, R. M., Jr., Runft, L., Kwan, K.,<br />
Haigo, S., Aronowicz, J., Lander, E., Gruber, C., Smith, M., Kirschner,<br />
M., and Gerhart, J. 2006. Dorsoventral patterning in hemichordates:<br />
insights into early chordate evolution. PLoS Biol 4:e291.<br />
Lowe, C. J., Wu, M., Salic, A., Evans, L., Lander, E., Stange-Thomann, N.,<br />
Gruber, C. E., Gerhart, J., and Kirschner, M. 2003. Anteroposterior<br />
patterning in hemichordates and the origins of the chordate nervous<br />
system. Cell 113:853-865.<br />
Lüter, C. 2000a. The origin of the coelom in Brachiopoda and its phylogenetic<br />
significance. Zoomorphology 120:15-28.<br />
Lüter, C. 2000b. Ultrastructure of larval and adult setae of Brachiopoda. Zool
References<br />
33<br />
Anz 239:75-90.<br />
Macdonald, P. M. and Struhl, G. 1986. A molecular gradient in early Drosophila<br />
embryos and its role in specifying the body pattern. Nature 324:537-<br />
545.<br />
Martindale, M. Q., Pang, K., and Finnerty, J. R. 2004. Investigating the origins<br />
of triploblasty: ‘mesodermal’ gene expression in a diploblastic animal,<br />
the sea anemone Nematostella vectensis (phylum, Cnidaria; class,<br />
Anthozoa). Development 131:2463-2474.<br />
Martinelli, C. and Spring, J. 2004. Expression pattern of the homeobox gene<br />
Not in the basal metazoan Trichoplax adhaerens. Gene Expr Patterns.<br />
4:443-447.<br />
McDougall, C., Chen, W.-C., Shimeld, S., and Ferrier, D. 2006. The development<br />
of the larval nervous system, musculature and ciliary bands of<br />
Pomatoceros lamarckii (Annelida): heterochrony in polychaetes. Front<br />
Zool 3:16.<br />
Meier, S. 1984. Somite formation and its relationship to metameric patterning of<br />
the mesoderm. Cell Differ 14:235-243.<br />
Morse, E. 1871. The Brachiopoda, a division of Annelida. In Proceedings of the<br />
American Association for Advances in Science, 19th meeting 1870.<br />
Morse, E. S. 1873. On the systematic position of the Brachiopoda. Boston:<br />
Press of A. A. Kingman.<br />
Nielsen, C. 1991. The development of the brachiopod Crania (Neocrania)<br />
anomala (O. F. Müller) and its phylogenetic significance. Acta Zool 72:7-<br />
28.<br />
Nielsen, C. 1995. Animal evolution: interrelationships of the living phyla. Oxford:<br />
Oxford University Press.<br />
Nielsen, C. and Worsaae, K. 2010. Structure and occurrence of cyphonautes<br />
larvae (Bryozoa, Ectoprocta). J Morphol 271:1094-1109.<br />
Noll, M. 1993. Evolution and role of Pax genes. Curr Opin Genet Dev 3:595-<br />
605.<br />
Orrhage, L. 1973. Light and electron microscope studies of some brachiopod<br />
and pogonophoran setae. Z. Morph. Tiere 74:253-270.<br />
Pires, A. and Woollacott, R. M. 1997. Serotonin and dopamine have opposite<br />
effects on phototaxis in larvae of the bryozoan Bugula neritina. Biol Bull<br />
192:399-409.<br />
Reed, C. G. and Cloney, R. A. 1977. Brachiopod tentacles - ultrastructure and<br />
functional significance of connective-tissue and myoepithelial cells in<br />
Terebratalia. Cell Tissue Res 185:17-42.<br />
Reichert, H. 2009. Evolutionary conservation of mechanisms for neural
34 References<br />
regionalization, proliferation and interconnection in brain development.<br />
Biol Lett 5:112-116.<br />
Richardson, J. R. and Watson, J. E. 1975. Locomotory adaptations in a freelying<br />
brachiopod. Science 189:381-382.<br />
Rieger, V., Perez, Y., Müller, C. H. G., Lipke, E., Sombke, A., Hansson, B. S., and<br />
Harzsch, S. 2010. Immunohistochemical analysis and 3D reconstruction<br />
of the cephalic nervous system in Chaetognatha: insights into the<br />
evolution of an early bilaterian brain? Invertebr Biol 129:77-104.<br />
Rowell, A. J. 1960. Some early stages in the development of the brachiopod<br />
Crania anomala (Müller). Ann. Mag. nat. Hist. 13:35-56.<br />
Rudwick, M. J. S. 1961. `Quick’ and `Catch’ adductor muscles in brachiopods.<br />
Nature 191:1021-1021.<br />
Rudwick, M. J. S. 1970. Living and fossil brachiopods. London: Hutchinson.<br />
Santagata, S. 2002. Structure and metamorphic remodeling of the larval<br />
nervous system and musculature of Phoronis pallida (Phoronida). Evol<br />
Dev 4:28-42.<br />
Santagata, S. and Zimmer, R. L. 2002. Comparison of the neuromuscular<br />
systems among actinotroch larvae: systematic and evolutionary<br />
implications. Evol Dev 4:43-54.<br />
Schmidt-Rhaesa, A. 2007a. Musculature. In The evolution of organ systems.<br />
Oxford: Oxford University Press.<br />
Schmidt-Rhaesa, A. 2007b. Nervous system. In The evolution of organ systems.<br />
Oxford: Oxford University Press.<br />
Scholtz, G. and Dohle, W. 1996. Cell lineage and cell fate in crustacean embryos<br />
- A comparative approach. Int J Dev Biol 40:211-220.<br />
Schram, F. R. 1991. Cladistic analysis of metazoan phyla and the placement of<br />
fossil Problematica. In The early evolution of Metazoa and the significance<br />
of problematic taxa, edited by A. M. Simonetta and S. Conway Morris:<br />
Cambridge University Press.<br />
Seaver, E. C., Thamm, K., and Hill, S. D. 2005. Growth patterns during<br />
segmentation in the two polychaete annelids, Capitella sp. I and<br />
Hydroides elegans: comparisons at distinct life history stages. Evol Dev<br />
7:312-326.<br />
Shimizu, K., Hunter, E., and Fusetani, N. 2000. Localisation of biogenic amines<br />
in larvae of Bugula neritina (Bryozoa: Cheilostomatida) and their effects<br />
on settlement. Mar Biol 136:1-9.<br />
Sommer, R. J. 2009. The future of evo-devo: model systems and evolutionary<br />
theory. Nat Rev Genet 10:416-422.<br />
Stricker, S. A. and Reed, C. G. 1985a. The ontogeny of shell secretion in
References<br />
35<br />
Terebratalia transversa (Brachiopoda, Articulata). 1. Development of the<br />
mantle. J Morphol 183:233-250.<br />
Stricker, S. A. and Reed, C. G. 1985b. The ontogeny of shell secretion in<br />
Terebratalia transversa (Brachiopoda, Articulata). 2. formation of the<br />
protegulum and juvenile shell. J Morphol 183:251-271.<br />
Telford, M. J. 2007. A single origin of the central nervous system? Cell 129:237-<br />
239.<br />
Thiemann, M. and Ruthmann, A. 1989. Microfilaments and microtubules in<br />
isolated fiber cells of Trichoplax adhaerens (Placozoa). Zoomorphology<br />
109:89-96.<br />
Trinh, L. A., McCutchen, M. D., Bonner-Fraser, M., Fraser, S. E., Bumm, L. A.,<br />
and McCauley, D. W. 2007. Fluorescent in situ hybridization employing<br />
the conventional NBT/BCIP chromogenic stain. Biotechniques 42:756-<br />
759.<br />
van Bemmelen, J. F. 1883. Untersuchungen über den anatomischen und<br />
histologischen Bau der Brachiopoda Testicardinia. Jen Zeit Nat 16:88-<br />
161.<br />
Venuti, J. M., Pepicelli, C., and Flowers, V. L. 2004. Analysis of sea urchin<br />
embryo gene expression by immunocytochemistry. Methods Cell Biol<br />
74:333-369.<br />
Voronezhskaya, E. E., Tsitrin, E. B., and Nezlin, L. P. 2003. Neuronal<br />
development in larval polychaete Phyllodoce maculata (Phyllodocidae).<br />
J Comp Neurol. 455:299-309.<br />
Wanninger, A. 2008. Comparative lophotrochozoan neurogenesis and larval<br />
neuroanatomy: Recent advances from previously neglected taxa. Acta<br />
Biol Hung 59:(Suppl.):127-136.<br />
Wanninger, A. 2009. Shaping the things to come: ontogeny of lophotrochozoan<br />
neuromuscular systems and the Tetraneuralia concept. Biol. Bull.<br />
216:293-306.<br />
Wanninger, A., Fuchs, J., and Haszprunar, G. 2007. Anatomy of the serotonergic<br />
nervous system of an entoproct creeping-type larva and its phylogenetic<br />
implications. Invertebr Biol 126:268-278.<br />
Watanabe, H., Fujisawa, T., and Holstein, T. W. 2009. Cnidarians and the<br />
evolutionary origin of the nervous system. Dev Growth Differ 51:167-<br />
183.<br />
Westfall, J. 1996. Ultrastructure of synapses in the first-evolved nervous<br />
systems. J Neurocytol 25:735-746.<br />
Westfall, J. A. and Elliott, C. F. 2002. Ultrastructure of the tentacle nerve plexus<br />
and putative neural pathways in sea anemones. Invertebr Biol 121:202-
36 References<br />
211.<br />
Williams, A., Carlson, S. J., Brunton, C. H. C., Holmer, L. E., and Popov, L.<br />
1996. A supra-ordinal classification of the Brachiopoda. Proc R Soc B<br />
351:1171-1193.<br />
Williams, A., James, M. A., Emig, C. C., Mackay, S., and Rhodes, M. C. 1997.<br />
Anatomy. In Treatise on Invertebrate Paleontology, Part H, Brachiopoda,<br />
Revised, edited by R. L. Kaesler. Lawrence, Kansas: Geological Society<br />
of America Inc. and The University of Kansas.<br />
Yatsu, N. 1902. On the development of Lingula anatina. J Coll Sci Tokyo 17:1-<br />
112.<br />
Younossi-Hartenstein, A., Green, P., Liaw, G.-J., Rudolph, K., Lengyel, J., and<br />
Hartenstein, V. 1997. Control of early neurogenesis of the Drosophila<br />
brain by the head gap genes tll, otd, ems,and btd. Dev Biol 182:270-<br />
283
Chapter II<br />
37<br />
Chapter II<br />
Altenburger, A. & Wanninger, A. 2009 Comparative larval<br />
myogenesis and adult myoanatomy of the rhynchonelliform<br />
(articulate) brachiopods Argyrotheca cordata, A. cistellula, and<br />
Terebratalia transversa. Frontiers in Zoology 6: 1-14
38 Chapter II<br />
Frontiers in Zoology<br />
BioMed Central<br />
Research<br />
Comparative larval myogenesis and adult myoanatomy of the<br />
rhynchonelliform (articulate) brachiopods Argyrotheca cordata, A.<br />
cistellula, and Terebratalia transversa<br />
Andreas Altenburger and Andreas Wanninger*<br />
Open Access<br />
Address: University of Copenhagen, Department of Biology, Research Group for Comparative Zoology, Universitetsparken 15, DK-2100<br />
Copenhagen Ø, Denmark<br />
Email: Andreas Altenburger - aaltenburger@bio.ku.dk; Andreas Wanninger* - awanninger@bio.ku.dk<br />
* Corresponding author<br />
Published: 3 February 2009<br />
Frontiers in Zoology 2009, 6:3<br />
doi:10.1186/1742-9994-6-3<br />
This article is available from: http://www.frontiersinzoology.com/content/6/1/3<br />
Received: 5 November 2008<br />
Accepted: 3 February 2009<br />
© 2009 Altenburger and Wanninger; licensee BioMed Central Ltd.<br />
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),<br />
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.<br />
Abstract<br />
Background: Despite significant methodological progress, Brachiopoda remains one of the<br />
lophotrochozoan phyla for which no recent ontogenetic data employing modern methodologies<br />
such as fluorescence labelling and confocal microscopy are available. This is particularly astonishing<br />
given the ongoing controversy concerning its phylogenetic position. In order to contribute new<br />
morphogenetic data for phylogenetic and evolutionary inferences, we describe herein the ontogeny<br />
and myoanatomy of larvae and adults of the rhynchonelliform brachiopods Argyrotheca cordata, A.<br />
cistellula, and Terebratalia transversa using fluorescence F-actin labelling combined with confocal<br />
laserscanning microscopy.<br />
Results: Fully grown larvae of A. cordata and T. transversa consist of three distinct body regions,<br />
namely an apical lobe, a mantle lobe with four bundles of setae, and a pedicle lobe. Myogenesis is<br />
very similar in these two species. The first anlagen of the musculature develop in the pedicle lobe,<br />
followed by setae muscles and the mantle lobe musculature. Late-stage larvae show a network of<br />
strong pedicle muscles, central mantle muscles, longitudinal muscles running from the mantle to<br />
the pedicle lobe, setae pouch muscles, setae muscles, a U-shaped muscle, serial mantle muscles,<br />
and apical longitudinal as well as apical transversal muscles. Fully developed A. cistellula larvae differ<br />
from the former species in that they have only two visible body lobes and lack setae. Nevertheless,<br />
we found corresponding muscle systems to all muscles present in the former two species, except<br />
for the musculature associated with the setae, in larvae of A. cistellula. With our survey of the adult<br />
myoanatomy of A. cordata and A. cistellula and the juvenile muscular architecture of T. transversa we<br />
confirm the presence of adductors, diductors, dorsal and ventral pedicle adjustors, mantle margin<br />
muscles, a distinct musculature of the intestine, and striated muscle fibres in the tentacles for all<br />
three species.<br />
Conclusion: Our data indicate that larvae of rhynchonelliform brachiopods share a common<br />
muscular bodyplan and are thus derived from a common ancestral larval type. Comparison of the<br />
muscular phenotype of rhynchonelliform larvae to that of the other two lophophorate phyla,<br />
Phoronida and Ectoprocta, does not indicate homology of individual larval muscles. This may be<br />
due to an early evolutionary split of the ontogenetic pathways of Brachiopoda, Phoronida, and<br />
Ectoprocta that gave rise to the morphological diversity of these phyla.<br />
Page 1 of 14<br />
(page number not for citation purposes)
Chapter II<br />
39<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
Background<br />
Brachiopoda is a small lophophorate phylum with a<br />
prominent fossil record since the Lower Cambrium [1].<br />
More than 12.000 fossil and approximately 380 recent<br />
species are known to date [2,3]. The phylum is commonly<br />
divided into three taxa, the articulate Rhynchonelliformea<br />
and the two inarticulate clades Craniiformea and Linguliformea<br />
[4], and has traditionally been grouped together<br />
with Phoronida and Ectoprocta into the superphylum<br />
Lophophorata. However, this classification has recently<br />
been challenged by paleontological and molecular datasets.<br />
While some analyses employing morphological data<br />
assign Brachiopoda to Deuterostomia [e.g., [5,6]], recent<br />
molecular data either propose sistergroup relationships to<br />
various spiralian phyla including Mollusca, Annelida, and<br />
Nemertea [7-11], or support the notion that Phoronida<br />
are an ingroup of Brachiopoda [12,13].<br />
Apart from some mainly gross morphological studies [14-<br />
21], detailed data using modern techniques such as fluorescence<br />
labelling and confocal laserscanning microscopy<br />
are not yet available. This is especially true with respect to<br />
the development of the musculature, despite the fact that<br />
myo-anatomical features may provide useful characters<br />
for reconstructing phylogenetic relationships [22,23].<br />
Recently, some data on larval muscle development for the<br />
proposed brachiopod sister groups Phoronida and Ectoprocta<br />
have become available [24-28]. Accordingly, larval<br />
myogenesis in Brachiopoda constitutes an important gap<br />
of knowledge in comparative developmental studies on<br />
Lophophorata. With the first thorough, comparative<br />
account of brachiopod larval myogenesis provided herein<br />
for the rhynchonelliform species Argyrotheca cordata<br />
(Risso, 1826), Argyrotheca cistellula (Searles-Wood, 1841),<br />
and Terebratalia transversa (Sowerby, 1846), we aim at<br />
stimulating the discussion concerning lophophorate bodyplan<br />
evolution, phylogeny, and development. Furthermore,<br />
we contribute to questions concerning the<br />
muscular ground pattern of rhynchonelliform brachiopod<br />
larvae. We supplement our ontogenetic data with a<br />
detailed description of the adult muscle systems of all<br />
three species.<br />
Results<br />
Embryonic and larval development of Argyrotheca<br />
cordata<br />
Embryos and larvae of Argyrotheca cordata are brooded by<br />
the mother animal and are released as late-stage larvae<br />
competent to undergo metamorphosis. Accordingly, larval<br />
development is entirely lecithotrophic. After cleavage<br />
and gastrulation (Fig. 1A), a three-lobed larva is established,<br />
which comprises an anterior apical lobe, a mantle<br />
lobe in the mid-body region, and a posterior pedicle lobe<br />
(Fig. 1B–F). In very early three-lobed stages, the blast-<br />
Scanning development Figure 1electron of Argyrotheca micrographs cordata of the embryonic and larval<br />
Scanning electron micrographs of the embryonic and<br />
larval development of Argyrotheca cordata. Anterior<br />
faces upward and scale bars equal 50 μm. (A) Early gastrula<br />
with blastopore (arrow). (B) Ventral view of an embryo at<br />
the onset of differentiation of the three-lobed larval bodyplan<br />
comprising apical lobe (AL), mantle lobe (ML), and pedicle<br />
lobe (PL). The arrowhead points to the region of the larval<br />
apical ciliary tuft. The arrow points to the larval mouth which<br />
corresponds to the blastopore. (C) Dorsal view of a larva<br />
with distinct anlagen of the three body lobes. (D) Ventral<br />
view of a specimen of the same ontogenetic stage as the one<br />
in C with reduced larval apical ciliary tuft (arrowhead) and<br />
with the almost closed blastopore (arrow). (E) Three-lobed<br />
larva at the onset of setae formation (double arrowheads),<br />
dorso-lateral view. (F) Lateral view of a fully differentiated<br />
larva showing two of the four pairs of larval setae (double<br />
arrowheads) and a distinct primordial hump (asterisk).<br />
Page 2 of 14<br />
(page number not for citation purposes)
40 Chapter II<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
opore is visible at the base of the apical lobe (Fig. 1B). This<br />
larval mouth closes during subsequent larval development<br />
(Fig. 1D).<br />
The apical lobe is ciliated and bears, in early three lobed<br />
stages, an apical tuft which is lost in later stages (Fig. 1B,<br />
D). When the three lobes are fully established, four bundles<br />
of larval setae are formed at the posterior margin of<br />
the mantle lobe (Fig. 1E). Finally, in larvae competent to<br />
undergo metamorphosis, the anlage of the pedicle<br />
becomes visible as a distinct primordial hump at the posterior<br />
pole of the pedicle lobe (Fig. 1F).<br />
Myogenesis and adult myoanatomy of Argyrotheca<br />
cordata<br />
The larvae investigated were about 230–270 μm long and<br />
210–240 μm wide. The first F-actin-positive signal is visible<br />
as distinct spots in the area that later forms the mantle<br />
lobe (Fig. 2A). These distinct spots are F-actin-positive<br />
microvilli which are situated in the lower part of the setal<br />
sacs where the setae are formed [cf. [29]]. The strong fluorescence<br />
signal of the microvilli disappears once setae formation<br />
is completed, due to the increasing predominance<br />
of the larval musculature (Fig. 2D–F).<br />
The pedicle muscles start to form in three-lobed larvae<br />
that still lack setae (Fig. 2B). In older larvae with short<br />
setae (corresponding to the stage shown in Fig. 1E), setae<br />
muscles start to develop. These run from the setal pouches<br />
in anterior direction and connect to the apical longitudinal<br />
muscles at the border between apical and mantle lobe<br />
(lateral setae muscles) or to the central mantle muscles<br />
(dorsal setae muscles), respectively (Fig. 2C). The apical<br />
longitudinal muscles extend laterally within the apical<br />
lobe and terminate anteriorly at an apical transversal muscle<br />
(Fig. 2C). At this stage, longitudinal muscles are also<br />
found within the pedicle lobe. From there, they run into<br />
the mantle lobe, where they connect to longitudinal muscles<br />
which originate at the muscle interconnection point<br />
at the border between apical and mantle lobe. The larval<br />
gut rudiment is visible as a tube in the centre of the larvae<br />
(Fig. 2C).<br />
In fully developed larvae, setae pouch muscles are established<br />
and interconnected by a circular mantle muscle<br />
(Fig. 2D). From this circular mantle muscle emerge serial<br />
mantle muscles, which are dorsolaterally closed by the<br />
central mantle muscles. The central mantle muscles are<br />
connected to the dorsal setae muscles and to the apical<br />
longitudinal muscles at the border of the apical and the<br />
mantle lobe (Fig. 2E–F). Anteroventrally, the serial mantle<br />
muscles are enclosed by a U-shaped muscle which extends<br />
ventrally from the pedicle muscles towards the circular<br />
mantle muscle (Fig. 2D–F; see also additional file 1). The<br />
primordial hump is devoid of any musculature (Fig. 2E–<br />
F).<br />
Adult A. cordata studied were 0.8–1.3 mm wide and 0.9–<br />
1.4 mm long. We can confirm four pairs of muscles which<br />
have been described previously [30]. These are one pair of<br />
adductors and one pair of diductors, which attach to both<br />
the dorsal and to the ventral valve. In addition, there are<br />
two pairs of pedicle adjustors, one of which being<br />
attached to the ventral valve and the pedicle, and one<br />
being attached to the dorsal valve and the pedicle (Fig.<br />
3A–B). In addition, we found a distinct musculature in the<br />
tentacles of the lophophore and in the digestive system.<br />
Each tentacle contains several bands of striated muscle<br />
fibers (Fig. 3D–E), while the stomach and intestine are<br />
each lined by numerous delicate ring muscles (Fig. 3C).<br />
Moreover, minute muscles are distributed along the dorsal<br />
and ventral mantle margin, which probably function<br />
as mantle retractor muscles. These mantle retractors are<br />
abundant and are oriented perpendicularly to the mantle<br />
margin that lines the shell (Fig. 3A–B).<br />
Myogenesis and adult myoanatomy of Argyrotheca<br />
cistellula<br />
Similar to Argyrotheca cordata, larvae of A. cistellula are lecithotrophic<br />
and are brooded by the mother animal. A. cistellula<br />
larvae lack setae and the mantle lobe encloses the<br />
pedicle lobe during development. Thus, the fully developed<br />
larvae have only two visible lobes, namely the apical<br />
and the mantle lobe. The investigated larvae were around<br />
117–139 μm long and 78–104 μm wide. The first muscles<br />
appear in larvae with all lobes fully differentiated. These<br />
are two dorsal mantle muscles which extend dorsally from<br />
anterior to posterior in the mantle lobe (Fig. 4A). Parallel<br />
and further lateral to these dorsal mantle muscles run the<br />
early lateral mantle muscles, and the first rudiments of the<br />
serial mantle muscles arise at this stage in the mantle lobe.<br />
These develop subsequently into a network of muscles<br />
that extends dorsally and ventrally from the two lateral<br />
mantle muscles (Fig. 4A–F). These lateral mantle muscles<br />
connect to the apical longitudinal muscles at the anterior<br />
pole and to the posterior muscle ring at the posterior pole<br />
of the larvae (Fig 4B–F). During subsequent development,<br />
the ventral mantle muscles and the pedicle muscles<br />
emerge (Fig. 4C). The pedicle muscles, situated in the centre<br />
of the mantle lobe, are the most prominent muscles in<br />
fully grown larvae (Fig. 4D). They connect to the apical<br />
longitudinal muscles, which in turn are in contact with<br />
the apical transversal muscles. The latter form a muscle<br />
ring in the apical lobe (Fig. 4E–F). The musculature of<br />
fully developed larvae includes the pedicle muscles, which<br />
are connected to the apical longitudinal muscles, the ventral<br />
mantle muscles, and the dorsal mantle muscles that<br />
connect to the pedicle muscles. Furthermore, serial mantle<br />
muscles, which extend dorsally and ventrally from the<br />
lateral mantle muscles, are present. Ventrally, the serial<br />
mantle muscles terminate at the ventral mantle muscles<br />
(Fig. 4F).<br />
Page 3 of 14<br />
(page number not for citation purposes)
Chapter II<br />
41<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
Myogenesis in Argyrotheca cordata<br />
Figure 2<br />
Myogenesis in Argyrotheca cordata. CLSM maximum projection micrographs, anterior faces upward. F-actin is labelled in<br />
red and cell nuclei are labelled in blue to indicate the outline of the specimens. Scale bars equal 50 μm. (A) Early larva in dorsal<br />
view with the first F-actin signals from microvilli (mi) within the setal canals. (B) Early three-lobed larval stage, postero-dorsal<br />
view, showing apical lobe (AL), mantle lobe (ML), pedicle lobe (PL), first rudiments of the pedicle musculature (pm), and microvilli<br />
(mi) in the setae pouches. (C) Larval stage with fully differentiated lobes and short setae in ventral view (corresponding to<br />
the larval stage shown in Fig. 1E). Visible are the apical transversal muscle (atm), the apical longitudinal muscles (alm), the interconnecting<br />
apical muscles (iam), the interconnecting mantle muscles (imm), the longitudinal muscles (lm), the foregut rudiment<br />
(fg), the hindgut rudiment (hg), the pedicle muscles (pm), microvilli (mi), the setae pouch musculature (arrowheads), and the<br />
setae muscles (sm). (D) Lateral right view of a fully developed three-lobed larva with the U-shaped muscle (empty arrows) on<br />
the ventral side. At this stage, the setae pouches are interconnected by a circular mantle muscle (arrow). New at this stage are<br />
the central mantle muscles (empty arrowhead). Further indicated are the setae pouch musculature (arrowheads), the setae<br />
muscles (sm), the serial mantle muscles (double arrowheads), the pedicle musculature (pm), and the apical longitudinal muscles<br />
(alm). (E) Same stage as in D, ventro-lateral view. The U-shaped muscle (empty arrows) is directly connected to the pedicle<br />
muscles (pm). In addition, the apical transversal muscle (atm), the apical longitudinal muscles (alm), the serial mantle muscles<br />
(double arrowhead), the central mantle muscles (empty arrowheads), the setae pouch muscles (arrowheads), the setae muscles<br />
(sm), the circular mantle muscle (arrow), and the primordial hump (asterisk) are indicated. (F) Fully grown larva in ventral<br />
view with circular mantle muscle (arrows), serial mantle muscles (double arrowheads), setae pouch muscles (arrowheads),<br />
setae muscles (sm), pedicle muscles (pm), longitudinal muscles (lm), apical longitudinal muscles (alm), apical transversal muscle<br />
(atm), interconnecting apical muscles (iam), primordial hump (asterisk), and central mantle muscles (empty arrowheads).<br />
Page 4 of 14<br />
(page number not for citation purposes)
42 Chapter II<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
Similar to the condition found in Argyrotheca cordata, four<br />
pairs of shell muscles are found in adult A. cistellula (Fig.<br />
5). One pair of shell adductors attaches medially to the<br />
dorsal and to the ventral valve (Fig. 5). Two pairs of pedicle<br />
adjustors extend posterior into the pedicle, whereby<br />
one attaches to the dorsal and one to the ventral valve.<br />
Finally, one pair of diductors attaches at the posterior end<br />
of the ventral valve and runs to the dorsal valve.<br />
Each tentacle of the lophophore contains a number of striated<br />
muscle fibres. Mantle margin muscles are arranged<br />
perpendicularly to the shell periphery along the edge of<br />
the dorsal and the ventral valve (Fig. 5A–B).<br />
Myogenesis, metamorphosis, and juvenile myoanatomy of<br />
Terebratalia transversa<br />
Larvae of Terebratalia transversa are lecithotrophic and<br />
develop for approximately four days at 11°C in the water<br />
column until they are competent to undergo metamorphosis.<br />
The investigated larvae were three-lobed, 120–178<br />
μm long and 94–141 μm wide, whereby the pedicle lobe<br />
was partly overgrown by the mantle lobe. The first developing<br />
muscles are the pedicle muscles and early rudiments<br />
of the serial mantle muscles (Fig. 6A). Thereafter,<br />
the musculature of the four setae pouches forms (Fig. 6B).<br />
In later stages, the setae pouch muscles interconnect with<br />
the circular mantle muscle (Fig. 6C). A U-shaped muscle<br />
extends on the ventral side of the larvae from the pedicle<br />
muscles towards the circular mantle muscle. The serial<br />
mantle muscles and the setae muscles span between the<br />
circular mantle muscle and the U-shaped muscle strand.<br />
The latter run from the setae pouches to the central mantle<br />
muscles (Fig. 6D). The central mantle muscles extend<br />
from the dorsal setae muscles, which run from the dorsal<br />
setae pouches towards the apical lobe. They connect to the<br />
apical longitudinal muscles at the border of the apical and<br />
the mantle lobe (Fig. 6D). Subsequently, the apical musculature<br />
develops, which consists of an apical transversal<br />
muscle and two lateral apical longitudinal muscles that<br />
are connected to the serial mantle muscles (Fig. 6E). In<br />
late three-lobed larvae, the pedicle muscles are, together<br />
with the central mantle muscles, the most prominent<br />
muscular structures. The central mantle muscles connect<br />
to the serial mantle muscles, the setae pouch muscles, the<br />
setae muscles, and the apical musculature (Fig. 6F).<br />
During metamorphosis, parts of the larval musculature<br />
appear to get resorbed and juvenile muscles develop (Fig.<br />
7A). We were, however, unable to clarify whether or not<br />
certain components of the larval musculature are incorporated<br />
into the juvenile muscular bodyplan.<br />
The juvenile musculature comprises early rudiments of<br />
the tentacle muscles, early rudiments of the mantle margin<br />
musculature, the musculature of the intestine, adductors,<br />
ventral pedicle adjustors which are connected to the<br />
diductors, and dorsal pedicle adjustors (Fig. 7B–D).<br />
Discussion<br />
Comparison of larval and adult rhynchonelliform<br />
myoanatomy<br />
The gross morphology of Argyrotheca cistellula differs considerably<br />
from that of A. cordata and Terebratalia transversa<br />
in that the pedicle lobe gets enclosed by the mantle lobe<br />
during development [19]. Thus, A. cistellula appears twolobed<br />
and lacks setae, while the other two species express<br />
three distinct body lobes and setae. Despite these differences,<br />
myogenesis follows a similar pattern in all three<br />
species (Table 1). When fully developed, prominent pedicle<br />
muscles, apical longitudinal as well as apical transversal<br />
muscles, and serial mantle muscles are present in all<br />
three species. In addition, A. cordata and T. transversa<br />
show a circular mantle muscle which we consider homologous<br />
to the posterior muscle ring in A. cistellula. This<br />
homology is based on the similar position of this muscle<br />
in the mantle lobe and the fact that the U-shaped muscle<br />
of A. cordata and T. transversa and the ventral mantle muscles<br />
of A. cistellula all insert at this muscle. The central<br />
mantle muscles of A. cordata and T. transversa are in our<br />
opinion homologous to the dorsal mantle muscles of A.<br />
cistellula due to the similar position of these muscles and<br />
their connection to the apical and the serial mantle muscles<br />
in all three species. The U-shaped muscle of A. cordata<br />
and T. transversa corresponds to the ventral mantle muscles<br />
in A. cistellula due to their similar position and the fact<br />
that these muscles enclose the serial mantle muscles<br />
antero-ventrally.<br />
Despite these similarities, we found distinct differences in<br />
the myoanatomy of the three species investigated. As<br />
such, the setae pouch muscles, the setae muscles, and the<br />
longitudinal muscles, which run from the mantle lobe to<br />
the pedicle lobe, are only present in A. cordata and T. transversa,<br />
while the lateral mantle muscles are only present in<br />
larvae of A. cistellula. These differences between A. cistellula<br />
on the one hand and A. cordata and T. transversa on<br />
the other correspond to the gross morphological observation<br />
that A. cistellula lacks setae.<br />
Larval setae in brachiopods have been proposed to function<br />
as a defence device and to control buoyancy [31]. The<br />
setae of A. cistellula larvae have probably been secondarily<br />
lost, as these larvae are brooded and may settle shortly<br />
after release from the mother animal. However, A. cordata<br />
larvae have retained their setae despite being brooded,<br />
which may hint towards an extended planktonic period of<br />
these larvae.<br />
The muscles in the pedicle lobe have been proposed earlier<br />
to be of functional use during metamorphosis<br />
Page 5 of 14<br />
(page number not for citation purposes)
Chapter II<br />
43<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
Adult myoanatomy of Argyrotheca cordata<br />
Figure 3<br />
Adult myoanatomy of Argyrotheca cordata. F-actin is labelled in red and cell nuclei are labelled in blue. Scale bars equal<br />
100 μm in all aspects except in E, where it equals 25 μm. (A) Overlay of CLSM maximum projection micrograph and light<br />
micrograph, anterior faces upward, dorsal view. Indicated are the tentacle muscles (tm), the mantle margin muscles (mm), the<br />
tentacles of the lophophore (te), the mantle cavity (mc), the intestine (in), the shell (s), the adductors (ad), the ventral pedicle<br />
adjustors (vpa), which extend from the ventral valve into the pedicle, the dorsal pedicle adjustors (dpa), which extend from the<br />
dorsal valve into the pedicle, and the diductors (di). One diductor is lacking as a result of the removal of the animal from the<br />
substrate. (B) Overlay of a CLSM maximum projection micrograph and a light micrograph, anterior faces upward, ventral view.<br />
Indicated are the same structures as in A. (C) Enlarged view of the ring musculature lining the intestine (in). In addition, one<br />
adductor (ad), the diductors (di), and the ventral pedicle adjustors (vpa) are visible. (D) Enlarged view of the tentacles of the<br />
lophophore and the corresponding tentacle musculature (tm). (E) Detail of a tentacle muscle fibre showing typical striation pattern<br />
(double arrows).<br />
Page 6 of 14<br />
(page number not for citation purposes)
44 Chapter II<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
[32,33]. When larvae settle, a glandular region at the tip of<br />
the primordial hump functions as site of attachment to<br />
the substrate [34]. Subsequently, the primordial hump<br />
forms the first rudiment of the juvenile pedicle. After larval<br />
settlement, the mantle lobe is inverted over the apical<br />
lobe and eventually forms the juvenile mantle. The apical<br />
lobe gets enclosed by the valves and forms the lophophore<br />
and all anterior adult structures [32,35]. At the<br />
onset of metamorphosis, the U-shaped muscle may, due<br />
to its connection to the pedicle muscles and the circular<br />
mantle muscle, aid in inverting the mantle lobe. During<br />
metamorphosis, the larval pedicle muscles are still present<br />
at the time of ventral pedicle adjustor and diductor formation.<br />
However, whether the larval pedicle muscles are<br />
resorbed or are (partly) incorporated into the juvenile<br />
diductor and/or pedal adjustor muscles could not be clarified<br />
by the present study.<br />
Argyrotheca cordata is the sole species from this study for<br />
which data on the larval myoanatomy had previously<br />
been available. In the first descriptions from 1873 and<br />
1883, "muscles abdominaux", that run from the pedicle<br />
lobe into the mantle lobe, had been identified [14,30]. A<br />
different description was given slightly later, when a network<br />
of muscles in the fully developed larva was<br />
described. The muscles were denoted "Muskel des lateralen<br />
Borstenbündels", "Muskel des medialen Borstenbündels",<br />
"musculus contractor", "musculus rotator<br />
dorsalis", and "musculus abductor" [15]. Our findings<br />
confirm the results of the first papers with respect to the<br />
pedicle muscles and the setae muscles. However, in our<br />
specimens, the pedicle muscles were not directly connected<br />
to the setae muscles as depicted in the first descriptions,<br />
but were instead connected to the U-shaped muscle.<br />
In adult Argyrotheca cordata, four pairs of muscles had<br />
been identified previously [30]. The pair of adductor muscles<br />
has two insertion sites, one anterior to the other at the<br />
dorsal valve, and an additional one at the ventral valve.<br />
The pair of diductor muscles inserts at the posterior part<br />
of both the ventral and the dorsal valve. One of the two<br />
pairs of adjustors inserts at the ventral valve and the pedicle,<br />
while the other pair inserts at the dorsal valve and the<br />
pedicle [30].<br />
The muscular systems of adult A. cordata and A. cistellula<br />
are similar to each other and comprise one pair of adductors,<br />
two pairs of pedicle adjustors and one pair of diductors.<br />
The tentacles contain several fibres of striated<br />
musculature which have previously been described as<br />
"rows of striated fusiform myoepithelial cells" in the<br />
lophophore of T. transversa [36].<br />
For the juvenile musculature of Terebratalia transversa we<br />
followed the nomenclature used by Eshleman and<br />
Wilkens [37]. The juvenile musculature, five days after<br />
metamorphosis, comprises rudiments of the tentacle<br />
muscles, rudiments of the mantle margin musculature,<br />
one pair of adductors, one pair of diductors, one pair of<br />
dorsal, and one pair of ventral pedicle adjustors. The ventral<br />
pedicle adjustors are connected to the diductors in the<br />
juvenile.<br />
Comparative myogenesis of Lophophorata<br />
For the Phoronida, data on muscle development are currently<br />
available for three species, namely Phoronis pallida,<br />
P. harmeri, and P. architecta [24,26,27]. The larvae of these<br />
species are of the actinotroch-type and differ considerably<br />
from brachiopod larvae in both their gross anatomy and<br />
in their lifestyle, because these phoronid larvae are planktotrophic,<br />
while the brachiopod larvae investigated herein<br />
are of the typical three-lobed, lecithotrophic type. Accordingly,<br />
a considerable part of the larval phoronid musculature<br />
is linked to the digestive system (e.g., the oesophageal<br />
ring muscles) and to the maintenance of a cylindrical<br />
body shape (e.g., a meshwork of circular and longitudinal<br />
muscles in the bodywall). In addition, trunk retractor<br />
muscles, that originate from the posterior collar ring muscles<br />
and insert in the telotrochal region, are present in<br />
phoronid larvae [27]. The collar region contains mainly<br />
ring muscles and few longitudinal muscles. The subumbrellar<br />
and exumbrellar layers of the hood contain circular<br />
muscles and a series of longitudinal muscles, which, in<br />
the exumbrellar layer, function as hood elevators [27].<br />
Furthermore, the tentacles of phoronid actinotroch larvae<br />
contain elevator and depressor muscles which consist of<br />
two loops in the elevators and a single loop in the depressors.<br />
These tentacle muscles are interconnected by the ring<br />
muscle of the collar [27]. We did not identify any muscles<br />
in the larvae of the three brachiopod species described<br />
herein that could potentially correspond to the actinotroch<br />
muscle systems known so far.<br />
The muscular architecture in ectoproct larvae is very<br />
diverse, thus following the high plasticity of larval gross<br />
morphology and the notion that lecithotrophic larvae<br />
might have evolved up to six times within Ectoprocta [38].<br />
To date, the larval muscular systems have been described<br />
for Membranipora membranacea (cyphonautes larva), Flustrellidra<br />
hispida (pseudocyphonautes larva), Celleporaria<br />
sherryae and Schizoporella floridana (both coronate larva),<br />
Bowerbankia gracilis (vesiculariform larva), Bugula stolonium<br />
and B. fulva (both buguliform larva), Sundanella<br />
sibogae, Nolella stipata, Amathia vidovici, Aeverrillia setigera,<br />
and Alcyonidium gelatinosum (all ctenostome larva), and<br />
Crisia elongata (cyclostome larva) [25,28]. Recently, a<br />
number of homologies have been proposed for various<br />
larval ectoproct muscle systems [25]. These are the coronal<br />
ring muscle, which underlies the ciliated, ring-shaped<br />
swimming organ of most larval types, the anterior median<br />
Page 7 of 14<br />
(page number not for citation purposes)
Chapter II<br />
45<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
Myogenesis in Argyrotheca cistellula<br />
Figure 4<br />
Myogenesis in Argyrotheca cistellula. Overlay of CLSM maximum projection micrograph and light micrograph, anterior<br />
faces upward. Scale bars equal 50 μm. Note that only two lobes are visible: the apical lobe (AL) and the mantle lobe (ML),<br />
which encloses the pedicle lobe. (A) Early larva in dorsal view with the dorsal mantle muscles (empty arrowheads), the early<br />
lateral mantle muscles (lmm), and early rudiments of the serial mantle muscles (double arrowheads). (B) Dorsal view of a later<br />
larval stage with the lateral mantle muscle strand (lmm), rudiments of the posterior muscle ring (arrow), dorsal mantle muscles<br />
(empty arrowheads), and the serial mantle muscles (double arrowhead). (C) Later larva in ventro-lateral left view with pedicle<br />
muscles (pm) that are connected to the ventral mantle muscles (empty arrows). The serial mantle muscles (double arrowhead)<br />
are connected to the lateral mantle muscles (lmm), the apical longitudinal muscles (alm) start to develop, and the early posterior<br />
muscle ring is visible (arrow). (D) Same stage as in C, dorsal view. The pedicle muscles (pm) are prominent and connect to<br />
the dorsal mantle muscles (empty arrowheads). In addition, the lateral mantle muscles (lmm), the serial mantle muscles (double<br />
arrowheads), a part of the posterior muscle ring (arrow), and the apical longitudinal muscles (alm) are visible. (E) Fully developed<br />
larva, ventral view. The apical transversal (atm) and the apical longitudinal muscles (alm) are fully developed and connect<br />
to the pedicle muscles (pm). The connection between pedicle muscles and dorsal mantle muscles (empty arrowheads) is visible<br />
in the anterior region of the pedicle muscles. Further indicated are the ventral mantle muscles (empty arrows), the serial mantle<br />
muscles (double arrowheads), the lateral mantle muscles (lmm), and the area of the posterior muscle ring (arrow). (F) Same<br />
larval stage as in E, ventro-lateral left view. The pedicle muscles (pm) are the most prominent muscles in the centre of the mantle<br />
lobe. They are connected to the apical longitudinal muscles (alm), which terminate at the apical transversal muscle (atm),<br />
which in turn forms a muscle ring in the apical lobe. The ventral mantle muscles (empty arrows) and dorsal mantle muscles<br />
(empty arrowhead) are also connected to the pedicle muscles. The serial mantle muscles (double arrowhead) extend dorsally<br />
and ventrally from the lateral mantle muscles (lmm). The latter terminate at the posterior muscle ring (arrow).<br />
Page 8 of 14<br />
(page number not for citation purposes)
46 Chapter II<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
muscle, which runs anteriorly from ventral to dorsal in<br />
most species, lateral muscles, which project laterally in<br />
dorso-ventral direction in most larvae, longitudinal muscles<br />
along the posterior body axis, and transversal muscles,<br />
which are situated transversally in the central body<br />
region of F. hispida, M. membranacea, and A. gelatinosum.<br />
Besides these proposed homologous muscles, each larval<br />
type shows unique muscles in the body wall and/or inside<br />
the larval body, reflecting at least partly the functional<br />
adaptations to a planktotrophic versus a lecithotrophic<br />
lifestyle. No muscles corresponding to any of the ectoproct<br />
muscle types were found in the brachiopod species<br />
investigated in this study (and noticeably no homologous<br />
muscles between the lecithotrophic ectoproct and brachiopod<br />
larval types could be identified), again demonstrating<br />
the high plasticity of lophophorate larval anatomy.<br />
Conclusion<br />
All rhynchonelliform brachiopod larvae studied to date<br />
are three-lobed with four bundles of setae [39], except for<br />
the larva of Argyrotheca cistellula, which is externally<br />
bilobed and lacks setae, and the three-lobed thecideid larvae,<br />
which likewise lack setae [40]. Despite these gross<br />
morphological differences, myogenesis in the three brachiopod<br />
species investigated is very similar. Thus, we propose<br />
a larval muscular groundpattern for<br />
rhynchonelliform brachiopods comprising apical longitudinal<br />
muscles, apical transversal muscles, circular mantle<br />
muscles, central mantle muscles, longitudinal muscles,<br />
serial mantle muscles, pedicle muscles, setae pouch muscles,<br />
setae muscles, and a U-shaped muscle. However, a<br />
final statement can only be made once data on the musculature<br />
of theceid and rhynchonellid larvae become<br />
available.<br />
Comparing this proposed larval muscular groundpattern<br />
to the hitherto investigated phoronids, ectoprocts, and<br />
spiralian taxa such as polychaetes, molluscs,<br />
plathelminths or entoprocts does not reveal any homologies<br />
of larval brachiopod muscles and the muscles of other<br />
lophotrochozoan larvae, regardless of whether the respective<br />
larvae are lecithotrophic or planktotrophic [23,41-<br />
47]. From these data we conclude that the ontogenetic<br />
pathways of the individual lophophorate phyla have split<br />
early in evolution from that of other Lophotrochozoa,<br />
which then resulted in the wide morphological diversity<br />
of larval and adult lophophorate bodyplans.<br />
Methods<br />
Animal collection and fixation<br />
Argyrotheca cordata and A. cistellula<br />
Adults were obtained from encrusting coralline red algae<br />
(coralligène), which was collected in the vicinity of the<br />
Observatoire Océanologique de Banyuls-sur-mer, France<br />
(42°29'27.51" N; 3°08'07.67" E), by SCUBA from 30–40<br />
m depth in July 2002 and June 2007. All developmental<br />
stages from unfertilized eggs to fully differentiated larvae<br />
were obtained by dissection from the adults. The specimens<br />
were relaxed at room temperature in 7.14% MgCl 2 ,<br />
fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate<br />
Adult myoanatomy of Argyrotheca cistellula<br />
Figure 5<br />
Adult myoanatomy of Argyrotheca cistellula. Overlay of CLSM maximum projection micrograph and light micrograph,<br />
anterior faces upward. Scale bars equal 300 μm. (A) Dorsal view. (B) Ventral view. Indicated are the mantle margin muscles<br />
(mm), the shell (s), the adductors (ad), the diductors (di), the dorsal pedicle adjustor (dpa), the ventral pedicle adjustor (vpa),<br />
the intestine (in), the mantle cavity (mc), and the tentacle muscles (tm).<br />
Page 9 of 14<br />
(page number not for citation purposes)
Chapter II<br />
47<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
Myogenesis in Terebratalia transversa<br />
Figure 6<br />
Myogenesis in Terebratalia transversa. Overlay of CLSM maximum projection micrograph and light micrograph, anterior<br />
faces upward. Scale bars equal 50 μm. (A) Ventral view of an early three-lobed stage with apical lobe (AL), mantle lobe (ML),<br />
and pedicle lobe (PL). Discernable are the pedicle musculature (pm), the first anlagen of the serial mantle muscles (double<br />
arrowhead), and the setae (se). (B) Ventral view of a slightly older larva with prominent pedicle musculature (pm), anlagen of<br />
the setae pouch musculature (arrowheads), and setae (se). (C) Later larval stage, ventral view with pedicle musculature (pm),<br />
setae pouch muscles (arrowhead), serial mantle muscles (double arrowhead), and central mantle muscles (empty arrowheads),<br />
which are extensions of the dorsal setae muscles. The serial mantle muscles are posteriorly connected to the circular mantle<br />
muscle (arrows) and antero-ventrally connected to the U-shaped muscle (empty arrows), which extends from the pedicle muscles<br />
to the circular mantle muscle. (D) Lateral view of a later larva with the muscle systems described in C. In addition, the first<br />
anlagen of the apical longitudinal musculature (alm), the setae muscles (sm), and the setae (se) are visible. (E) Same stage as in<br />
D with prominent pedicle muscles (pm) that are connected to the apical longitudinal muscles (alm). The latter connect to the<br />
apical transversal muscle (atm). In addition, the setae pouch muscles (arrowheads), the setae muscles (sm), and the setae (se)<br />
are indicated. (F) Fully developed larva, ventral view, with central mantle muscles (empty arrowheads), pedicle muscles (pm),<br />
circular mantle muscle (arrows), U-shaped muscle (empty arrows), serial mantle muscles (double arrowheads), setae pouch<br />
musculature (arrowheads), setae muscles (sm), apical longitudinal muscles (alm), apical transversal muscle (atm), and setae (se).<br />
Page 10 of 14<br />
(page number not for citation purposes)
48 Chapter II<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
Metamorphosis and adult myoanatomy of Terebratalia transversa<br />
Figure 7<br />
Metamorphosis and adult myoanatomy of Terebratalia transversa. (A-C) Overlay of CLSM maximum projection<br />
micrograph and light micrograph, anterior faces upward. F-actin is labelled in red and cell nuclei are labelled in blue. Scale bars<br />
equal 50 μm. (A) Larva during metamorphosis. A mosaic of larval and juvenile features are present including the pedicle (pe),<br />
the larval pedicle muscles (pm), the first rudiments of the juvenile tentacle musculature (tm), one diductor (di), and the ventral<br />
pedicle adjustors (vpa). (B) Juvenile 5 days after metamorphosis, dorsal view with the remaining larval setae (se), the mantle<br />
margin muscles (mm), the tentacle muscles (tm), the adductors (ad), the musculature of the intestine (in), the diductors (di),<br />
the ventral pedicle adjustors (vpa), the dorsal pedicle adjustors (dpa), and the pedicle (pe). (C) Juvenile 5 days after metamorphosis,<br />
ventral view with the remaining larval setae (se), rudiments of the mantle margin muscles (mm), rudiments of the tentacle<br />
muscles (tm), the adductors (ad), the ventral pedicle adjustors (vpa), the diductors (di), the dorsal pedicle adjustors (dpa),<br />
and the pedicle (pe). (D) Reconstruction of the 3D arrangement of the juvenile musculature based on the CLSM dataset used<br />
in C showing the dorsal pedicle adjustors (red), the adductors (dark blue), the mantle margin muscles (light blue), and the tentacle<br />
muscles (magenta). The ventral pedicle adjustors (yellow) are ventrally connected to the diductors (green).<br />
Page 11 of 14<br />
(page number not for citation purposes)
Chapter II<br />
49<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
buffer (PB) for 2 hours or for 3–5 hours, and subsequently<br />
washed thrice with 0.1 M PB for 15 min each. The samples<br />
were stored in 0.1 M PB with 0.1% NaN 3 at 4°C. Material<br />
fixed for 2 hours was used for immunocytochemistry<br />
(ICC) and material fixed for 3–5 hours was used for scanning<br />
electron microscopy (SEM).<br />
Terebratalia transversa<br />
Adults were collected in the San Juan Archipelago, USA, in<br />
the vicinity of the Friday Harbor Laboratories, and were<br />
kept in running seawater tables. To obtain larvae, females<br />
were dissected and their eggs transferred into beaker<br />
glasses with filtered seawater. The seawater was changed<br />
several times in order to wash off follicle cells, and the<br />
eggs were left overnight for germinal vesicle breakdown.<br />
Males were opened and left in filtered seawater overnight.<br />
Thereafter, their testes were scraped out, macerated, and<br />
diluted with filtered seawater to obtain a sperm suspension.<br />
Prior to fertilization, sperm cells were activated by<br />
adding three drops of a 1 M Tris buffer solution (Sigma-<br />
Aldrich, St. Louis, MO, USA) to approximately 50 ml of<br />
sperm suspension. Larvae were maintained in embryo<br />
dishes at around 11°C and the filtered seawater was<br />
changed twice daily. Free swimming larvae, metamorphic<br />
stages, and juveniles five days after metamorphosis were<br />
relaxed in 7.14% MgCl 2 and fixed in 4% PFA in 0.1 M PB<br />
for 30 min at room temperature. Larvae were washed<br />
thrice for 15 min in 0.1 M PB and stored in 0.1 M PB with<br />
0.1% NaN 3 at 4°C.<br />
Scanning electron microscopy<br />
For scanning electron microscopy (SEM), the specimens<br />
were postfixed in 1% OsO 4 , dehydrated in a graded acetone<br />
series, critical point dried, and sputter coated with<br />
gold. Digital images were acquired using a LEO 1430 VP<br />
SEM (Zeiss, Jena, Germany).<br />
Table 1: Comparative larval myoanatomy of the rhynchonelliform brachiopods Argyrotheca cordata, Terebratalia transversa, and A.<br />
cistellula<br />
Species<br />
Muscle Argyrotheca cordata Terebratalia<br />
transversa<br />
Argyrotheca<br />
cistellula<br />
Location<br />
Symbol in figures<br />
apical longitudinal<br />
muscles<br />
apical transversal<br />
muscle<br />
+ + + apical lobe alm<br />
+ + + (apical muscle ring) apical lobe atm<br />
central mantle muscles + + +<br />
(dorsal mantle<br />
muscles)<br />
mantle lobe<br />
empty arrowheads<br />
circular mantle muscle + + +<br />
(posterior muscle ring)<br />
mantle lobe<br />
arrows<br />
lateral mantle muscle - - + mantle lobe lmm<br />
longitudinal muscles + + - mantle and pedicle<br />
lobe<br />
lm<br />
pedicle muscles + + + pedicle lobe pm<br />
serial mantle muscles + + + mantle lobe double arrowsheads<br />
setae muscles + + - mantle lobe sm<br />
setae pouch<br />
musculature<br />
+ + - mantle lobe arrowheads<br />
U-shaped muscle + + +<br />
(ventral mantle<br />
muscle)<br />
mantle lobe<br />
empty arrows<br />
Page 12 of 14<br />
(page number not for citation purposes)
50 Chapter II<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
F-actin labelling, confocal laserscanning microscopy<br />
(CLSM), and 3D reconstruction<br />
Prior to staining, larvae were washed thrice for 15 min in<br />
PB and incubated for 1 h in PB containing 0.1% Triton X-<br />
100 (Sigma-Aldrich) to permeabilize the tissue. Then, the<br />
specimens were incubated in 1:40 diluted Alexa Fluor 488<br />
phalloidin (Invitrogen, Molecular Probes, Eugene, OR,<br />
USA) and 3 μg/ml DAPI (Invitrogen) in the permeabilization<br />
solution overnight at 4°C. Subsequently, specimens<br />
were washed thrice for 15 min in 0.1 M PB and embedded<br />
in Fluoromount G (Southern Biotech, Birmingham, AL,<br />
USA) on glass slides. The same procedure was used for<br />
juveniles and adults, with the addition of a decalcifying<br />
step using 0.05 M EGTA (Sigma-Aldrich) at room temperature<br />
overnight prior to permeabilization and staining.<br />
Negative controls omitting the phalloidin dye were performed<br />
on all species in order to avoid potential misinterpretations<br />
caused by autofluorescence.<br />
The samples were analysed with a Leica DM RXE 6 TL fluorescence<br />
microscope equipped with a TCS SP2 AOBS<br />
laserscanning device (Leica Microsystems, Wetzlar, Germany).<br />
Animals were scanned at intervals of 0.49 μm or<br />
0.64 μm, respectively, and the resulting image stacks were<br />
merged into maximum projection images. Photoshop<br />
CS3 (Adobe, San Jose, CA, USA) was used to create overlay<br />
images of CLSM and light micrographs and for assembling<br />
the figure plates. 3D reconstruction was performed<br />
on CLSM datasets using volume rendering algorithms of<br />
the graphics software Imaris 5.7.2 (Bitplane, Zurich, Switzerland).<br />
Competing interests<br />
The authors declare that they have no competing interests.<br />
Authors' contributions<br />
AA performed research and drafted the manuscript. AW<br />
designed and coordinated research, performed the SEM<br />
analysis, and contributed significantly to the writing of<br />
the manuscript. Both authors read and approved the final<br />
version of the manuscript.<br />
Additional material<br />
Additional file 1<br />
Larval musculature of Argyrotheca cordata. Movie of a confocal scan<br />
through a fully developed larva of Argyrotheca cordata to illustrate the<br />
three-dimensional arrangement of the larval musculature.<br />
Click here for file<br />
[http://www.biomedcentral.com/content/supplementary/1742-<br />
9994-6-3-S1.mpg]<br />
Acknowledgements<br />
We are grateful to Henrike Semmler (Copenhagen) for rearing and fixing<br />
Terebratalia larvae during the Comparative Invertebrate Embryology class<br />
2006 at the Friday Harbor Laboratories and for comments on an early draft<br />
of the manuscript. We further thank the divers and the staff of the Marine<br />
Biological Station Banyuls-sur-mer for collecting the coralligène and for<br />
providing laboratory space. Scott Santagata (Brookville, New York) is<br />
thanked for comments on the manuscript and Jana Hoffmann (Berlin, Germany)<br />
for providing access to some of the classic literature. The valuable<br />
comments of an anonymous reviewer helped to improve the manuscript.<br />
This study was funded by the Danish Agency for Science, Technology and<br />
Innovation (grant no. 645-06-0294 to AW) and the Danish Research<br />
Agency (grant no. 21-04-0356 to AW). Research in the lab of A. Wanninger<br />
is further supported by the EU-funded Marie Curie Network MOLMORPH<br />
(contract grant number MEST-CT-2005-020542).<br />
References<br />
1. Williams A, Rowell AJ: Evolution and Phylogeny. In Treatise on<br />
Invertebrate Paleontology, Part H, Brachiopoda Volume 1. Edited by:<br />
Moore RC. Lawrence, Kansas: The Geological Society of America and<br />
The University of Kansas; 1965:164-199.<br />
2. Logan A: Geographic distribution of extant articulated brachiopods.<br />
In Treatise on Invertebrate Paleontology, Part H, Brachiopoda,<br />
Revised Volume 6. Edited by: Selden PA. Boulder, Colorado, and Lawrence,<br />
Kansas: The Geological Society of America, Inc. and The University<br />
of Kansas; 2007:3082-3115.<br />
3. Stechmann A, Schlegel M: Analysis of the complete mitochondrial<br />
DNA sequence of the brachiopod Terebratulina retusa<br />
places Brachiopoda within the protostomes. Proc R Soc Lond B<br />
Biol Sci 1999, 266:2043-2052.<br />
4. Williams A, Carlson SJ, Brunton CHC, Holmer LE, Popov L: A supraordinal<br />
classification of the Brachiopoda. Proc R Soc Lond B Biol<br />
Sci 1996, 351:1171-1193.<br />
5. Nielsen C: The phylogenetic position of Entoprocta, Ectoprocta,<br />
Phoronida, and Brachiopoda. Integr Comp Biol 2002,<br />
42:685-691.<br />
6. Lüter C, Bartolomaeus T: The phylogenetic position of Brachiopoda<br />
– a comparison of morphological and molecular data.<br />
Zool Scr 1997, 26:245-253.<br />
7. Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA,<br />
Seaver E, Rouse GW, Obst M, Edgecombe GD, et al.: Broad phylogenomic<br />
sampling improves resolution of the animal tree of<br />
life. Nature 2008, 452:745-749.<br />
8. Helmkampf M, Bruchhaus I, Hausdorf B: Multigene analysis of<br />
lophophorate and chaetognath phylogenetic relationships.<br />
Mol Phylogenet Evol 2008, 46:206-214.<br />
9. Mallatt J, Winchell CJ: Testing the new animal phylogeny: First<br />
use of combined large-subunit and small-subunit rRNA gene<br />
sequences to classify the protostomes. Mol Biol Evol 2002,<br />
19:289-301.<br />
10. Passamaneck Y, Halanych KM: Lophotrochozoan phylogeny<br />
assessed with LSU and SSU data: Evidence of lophophorate<br />
polyphyly. Mol Phylogenet Evol 2006, 40:20-28.<br />
11. Peterson KJ, Eernisse DJ: Animal phylogeny and the ancestry of<br />
bilaterians: inferences from morphology and 18S rDNA gene<br />
sequences. Evol Dev 2001, 3:170-205.<br />
12. Cohen BL: Monophyly of brachiopods and phoronids: reconciliation<br />
of molecular evidence with Linnaean classification<br />
(the subphylum Phoroniformea nov.). Proc R Soc Lond B Biol Sci<br />
2000, 267:225-231.<br />
13. Cohen BL, Weydmann A: Molecular evidence that phoronids<br />
are a subtaxon of brachiopods (Brachiopoda: Phoronata)<br />
and that genetic divergence of metazoan phyla began long<br />
before the early Cambrian. Org Divers Evol 2005, 5:253-273.<br />
14. Kowalevski AO: Observations sur le développement des brachiopodes<br />
(Analyse par Oehlert et Deniker). Arch Zool Exp Gen<br />
1883, Series 2:57-76.<br />
Page 13 of 14<br />
(page number not for citation purposes)
Chapter II<br />
51<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
15. Plenk H: Die Entwicklung von Cistella (Argiope) neapolitana.<br />
Ein Beitrag zur Entwicklungsgeschichte der Brachiopoden<br />
(1. Mitteilung). Arb zool Inst Univ Wien 1913, 20:93-108.<br />
16. Chuang SH: The embryonic, larval and early postlarval development<br />
of the terebratellid brachiopod Calloria inconspicua<br />
(Sowerby). J R Soc NZ 1996, 26:119-137.<br />
17. Long JA: The embryology of three species representing three<br />
superfamilies of articulate Brachiopoda. University of Washington,<br />
<strong>PhD</strong> Thesis; 1964.<br />
18. Lüter C: Embryonic and larval development of Calloria inconspicua<br />
(Brachiopoda, Terebratellidae). J R Soc NZ 1998,<br />
28:165-167.<br />
19. Grobe P, Lüter C: Reproductive cycles and larval morphology<br />
of three Recent species of Argyrotheca (Terebratellacea: Brachiopoda)<br />
from Mediterranean submarine caves. Mar Biol<br />
1999, 134:595-600.<br />
20. Lüter C: Anatomy. In Treatise on Invertebrate Paleontology, Part H,<br />
Brachiopoda, Revised Volume 6. Edited by: Selden PA. Boulder, Colorado,<br />
and Lawrence, Kansas: The Geological Society of America, Inc.<br />
and The University of Kansas; 2007:2321-2355.<br />
21. Williams A, Rowell AJ: Brachiopod Anatomy. In Treatise on Invertebrate<br />
Paleontology, Part H, Brachiopoda Volume 1. Edited by: Moore<br />
RC. Lawrence, Kansas: Geological Society of America and University<br />
of Kansas Press; 1965:6-57.<br />
22. Hooge MD, Tyler S: Concordance of molecular and morphological<br />
data: The example of the Acoela. Integr Comp Biol 2006,<br />
46:118-124.<br />
23. Wanninger A: Myo-anatomy of juvenile and adult loxosomatid<br />
entoprocta and the use of muscular body plans for phylogenetic<br />
inferences. J Morphol 2004, 261:249-257.<br />
24. Santagata S: Structure and metamorphic remodeling of the<br />
larval nervous system and musculature of Phoronis pallida<br />
(Phoronida). Evol Dev 2002, 4:28-42.<br />
25. Gruhl A: Muscular systems in gymnolaemate bryozoan larvae<br />
(Bryozoa: Gymnolaemata). Zoomorphology 2008, 127:143-159.<br />
26. Santagata S: Larval development of Phoronis pallida (Phoronida):<br />
Implications for morphological convergence and divergence<br />
among larval body plans. J Morphol 2004, 259:347-358.<br />
27. Santagata S, Zimmer RL: Comparison of the neuromuscular systems<br />
among actinotroch larvae: systematic and evolutionary<br />
implications. Evol Dev 2002, 4:43-54.<br />
28. Santagata S: The morphology and evolutionary significance of<br />
the ciliary fields and musculature among marine bryozoan<br />
larvae. J Morphol 2008, 269:349-364.<br />
29. Lüter C: Ultrastructure of larval and adult setae of Brachiopoda.<br />
Zool Anz 2000, 239:75-90.<br />
30. Shipley AE: On the structure and development of Argiope. Mitt<br />
zool Stat Neapel 1883, 4:494-520.<br />
31. Chuang SH: Larval development in Discinisca (inarticulate brachiopod).<br />
Am Zool 1977, 17:39-53.<br />
32. Stricker SA, Reed CG: The ontogeny of shell secretion in Terebratalia<br />
transversa (Brachiopoda, Articulata). 1. Development<br />
of the mantle. J Morphol 1985, 183:233-250.<br />
33. Lüter C: Zur Ultrastruktur, Ontogenese und Phylogenie der Brachiopoda<br />
Göttingen: Cuvillier Verlag; 1998.<br />
34. James MA, Ansell AD, Collins MJ, Curry GB, Peck LS, Rhodes MC:<br />
Biology of living brachiopods. Adv Mar Biol 1992, 28:175-387.<br />
35. Stricker SA, Reed CG: The ontogeny of shell secretion in Terebratalia<br />
transversa (Brachiopoda, Articulata). 2. Formation of<br />
the protegulum and juvenile shell. J Morphol 1985, 183:251-271.<br />
36. Reed CG, Cloney RA: Brachiopod tentacles – ultrastructure<br />
and functional significance of connective-tissue and myoepithelial<br />
cells in Terebratalia. Cell Tissue Res 1977, 185:17-42.<br />
37. Eshleman WP, Wilkens JL: Actomyosin ATPase activities in the<br />
brachiopod Terebratalia transversa. Can J Zool 1979,<br />
57:1944-1949.<br />
38. Strathmann RR: Evolution and loss of feeding larval stages of<br />
marine invertebrates. Evolution 1978, 32:894-906.<br />
39. Pennington JT, Stricker SA: Phylum Brachiopoda. In Atlas of<br />
Marine Invertebrate Larvae Edited by: Young CM. San Diego: Academic<br />
Press; 2002:441-461.<br />
40. de Lacaze-Duthiers FJH: Histoire naturelle des brachiopodes<br />
vivants de la Mediterranée. I. Histoire naturelle de la Thecidie<br />
(Thecidium mediterraneum). Ann Sci Nat Zool (serie 4) 1861,<br />
15:259-330.<br />
41. Hill SD, Boyer BC: Phalloidin labeling of developing muscle in<br />
embryos of the polychaete Capitella sp. I. Biol Bull 2001,<br />
201:257-258.<br />
42. Reiter D, Boyer B, Ladurner P, Mair G, Salvenmoser W, Rieger R:<br />
Differentiation of the body wall musculature in Macrostomum<br />
hystricinum marinum and Hoploplana inquilina<br />
(Plathelminthes) as models for muscle development in<br />
lower Spiralia. Dev Genes Evol 1996, 205:410-423.<br />
43. Fuchs J, Bright M, Funch P, Wanninger A: Immunocytochemistry<br />
of the neuromuscular systems of Loxosomella vivipara and<br />
Loxosomella parguerensis (Entoprocta: Loxosomatidae). J<br />
Morphol 2006, 267:866-883.<br />
44. McDougall C, Chen W-C, Shimeld S, Ferrier D: The development<br />
of the larval nervous system, musculature and ciliary bands<br />
of Pomatoceros lamarckii (Annelida): heterochrony in polychaetes.<br />
Front Zool 2006, 3:16.<br />
45. Nielsen C, Haszprunar G, Ruthensteiner B, Wanninger A: Early<br />
development of the aplacophoran mollusc Chaetoderma.<br />
Acta Zool 2007, 88:231-247.<br />
46. Wanninger A, Haszprunar G: Chiton myogenesis: Perspectives<br />
for the development and evolution of larval and adult muscle<br />
systems in molluscs. J Morphol 2002, 251:103-113.<br />
47. Wanninger A, Ruthensteiner B, Lobenwein S, Salvenmoser W, Dictus<br />
WJAG, Haszprunar G: Development of the musculature in the<br />
limpet Patella (Mollusca, Patellogastropoda). Dev Genes Evol<br />
1999, 209:226-238.<br />
Publish with BioMed Central and every<br />
scientist can read your work free of charge<br />
"BioMed Central will be the most significant development for<br />
disseminating the results of biomedical research in our lifetime."<br />
Sir Paul Nurse, Cancer Research UK<br />
Your research papers will be:<br />
available free of charge to the entire biomedical community<br />
peer reviewed and published immediately upon acceptance<br />
cited in PubMed and archived on PubMed Central<br />
yours — you keep the copyright<br />
Submit your manuscript here:<br />
http://www.biomedcentral.com/info/publishing_adv.asp<br />
BioMedcentral<br />
Page 14 of 14<br />
(page number not for citation purposes)
52 Chapter III<br />
Chapter III<br />
Altenburger, A. & Wanninger, A. 2010 Neuromuscular development<br />
in Novocrania anomala: evidence for the presence of serotonin<br />
and a spiralian-like apical organ in lecithotrophic brachiopod<br />
larvae. Evolution & Development 12: 16-24
Chapter III<br />
53<br />
EVOLUTION & DEVELOPMENT 12:1, 16–24 (2010)<br />
DOI: 10.1111/j.1525-142X.2009.00387.x<br />
Neuromuscular development in Novocrania anomala: evidence for the<br />
presence of serotonin and a spiralian-like apical organ in lecithotrophic<br />
brachiopod larvae<br />
Andreas Altenburger and Andreas Wanninger <br />
Department of Biology, Research Group for Comparative Zoology, University of Copenhagen, Universitetsparken 15,<br />
DK-2100 Copenhagen Ø, Denmark<br />
Author for correspondence (email: awanninger@bio.ku.dk)<br />
SUMMARY The phylogenetic position of Brachiopoda remains<br />
unsettled, and only few recent data on brachiopod<br />
organogenesis are currently available. In order to contribute<br />
data to questions concerning brachiopod ontogeny and evolution<br />
we investigated nervous and muscle system development<br />
in the craniiform (inarticulate) brachiopod Novocrania<br />
anomala. Larvae of this species are lecithotrophic and have<br />
a bilobed body with three pairs of dorsal setal bundles that<br />
emerge from the posterior lobe. Fully developed larvae exhibit<br />
a network of setae pouch muscles as well as medioventral<br />
longitudinal and transversal muscles. After settlement, the<br />
anterior and posterior adductor muscles and delicate mantle<br />
retractor muscles begin to form. Comparison of the larval<br />
muscular system of Novocrania anomala with that of<br />
rhynchonelliform (articulate) brachiopod larvae shows that<br />
the former has a much simpler muscular organization. The<br />
first signal of serotonin-like immunoreactivity appears in fully<br />
developed Novocrania anomala larvae, which have an apical<br />
organ that consists of four flask-shaped cells and two ventral<br />
neurites. These ventral neurites do not stain positively for the<br />
axonal marker a-tubulin in the larval stages. In the juveniles,<br />
the nervous system stained by a-tubulin is characterized by<br />
two ventral neurite bundles with three commissures. Our data<br />
are the first direct proof for the presence of an immunoreactive<br />
neurotransmitter in lecithotrophic brachiopod larvae and<br />
demonstrate the existence of flask-shaped serotonergic cells<br />
in the brachiopod larval apical organ, thus significantly<br />
increasing the probability that this cell type was part of the<br />
bauplan of the larvae of the last common lophotrochozoan<br />
ancestor.<br />
INTRODUCTION<br />
The phylogenetic position of Brachiopoda remains unresolved,<br />
although most molecular analyses agree on their inclusion<br />
within Lophotrochozoa (Hejnol et al. 2009; Paps et al.<br />
2009). Alternatively, some recent works support the more<br />
traditional view that Brachiopoda clusters with Ectoprocta<br />
and Phoronida to form the Lophophorata, the direct sistergroup<br />
of Spiralia (Trochozoa) (Gee 1995; Nielsen 2002; Halanych<br />
2004). Current brachiopod internal phylogeny suggests<br />
division of the phylum into the three clades Linguliformea,<br />
Craniiformea, and Rhynchonelliformea (Williams et al. 1996).<br />
Craniiform brachiopods share morphological traits with both<br />
linguliforms and rhynchonelliforms. For example, craniiforms<br />
and linguliforms possess a circumferential mantle cavity, a<br />
muscle system with oblique muscles, and two pairs of shell<br />
adductors, a transitional median tentacle during lophophore<br />
development and a median division of the brachial canals into<br />
two separate cavities within the lophophore. Craniiforms and<br />
rhynchonelliformes exhibit a proteinaceous calcitic shell, a<br />
16<br />
single row of tentacles on a trocholophous lophophore,<br />
gonads suspended in the mantle sinus, and lecithotrophic<br />
larvae (Rowell 1960; Atkins and Rudwick 1962; Williams<br />
et al. 1996).<br />
Experimental embryology has shown that the animal half<br />
of the egg forms the ectodermal epithelium of the apical lobe,<br />
whereas the vegetal half forms endoderm, mesoderm, and the<br />
ectoderm of the mantle lobe in Novocrania anomala (Mu¨ ller<br />
1776) (previously assigned to various genera and thus also<br />
referred to in the literature as Crania anomala or Neocrania<br />
anomala, respectively) (Lee and Brunton 1986, 2001; Freeman<br />
and Lundelius 1999; Freeman 2000; Holmer 2001; Cohen et<br />
al. 2008). During metamorphosis, both the ventral and the<br />
dorsal valve are formed from the dorsal epithelium of the<br />
larva (Nielsen 1991).<br />
Recent immunocytochemical studies have revealed the almost<br />
universal occurrence of an apical organ that contains<br />
flask-shaped cells in larvae of Annelida, Mollusca, Sipuncula,<br />
Entoprocta, and Platyhelminthes (see Wanninger 2009 for<br />
review). These flask-shaped cells express serotonin-like<br />
& 2010 Wiley Periodicals, Inc.
54 Chapter III<br />
Altenburger and Wanninger<br />
immunoreactivity and may also show FMRFamidergic<br />
immunoreactivity. The wide occurrence of serotonin indicates<br />
that this neurotransmitter was part of the ancestral metazoan<br />
nervous system (Hay-Schmidt 2000). Surprisingly, neither<br />
serotonin-like immunoreactivity nor the existence of flaskshaped<br />
cells have hitherto been proven for lecithotrophic larvae<br />
of any brachiopod clade, thus leaving a significant gap in<br />
our understanding of the evolution of the brachiopod nervous<br />
system and the origin of this cell type within the lophophorates.<br />
Accordingly, we provide herein the first thorough<br />
immunocytochemical study on neurogenesis in a brachiopod<br />
with a lecithotrophic larva, the craniiform Novocrania anomala,<br />
and compare our findings with data on other lophotrochozoan<br />
phyla. In our general quest to shed light on<br />
brachiopod organogenesis, we also present data on Novocrania<br />
anomala myogenesis, which for the first time allows conclusive<br />
comparisons between the muscular systems of<br />
craniiform and rhynchonelliform brachiopod larvae and thus<br />
contributes to answering questions concerning the ancestral<br />
muscular bodyplan of brachiopod larvae.<br />
MATERIALS AND METHODS<br />
Animal collection, breeding, and fixation<br />
Rocks with attached adults of Novocrania anomala where obtained<br />
by dredging in the vicinity of the Sven Love´ n Centre for Marine<br />
Sciences, Gullmarsfjord, Sweden (58115 0 921 00 N, 11125 0 103 00 E) in<br />
October 2007 and September 2008. The rocks were maintained in<br />
the laboratory in running seawater and adults were removed and<br />
dissected for gametes. For artificial fertilization, eggs and sperm<br />
were removed from the gonads with pulled glass pipettes and<br />
placed in separate glass beakers with filtered seawater at ambient<br />
seawater temperature (141C). The water containing the eggs was<br />
changed at least four times to wash off follicle cells and superfluous<br />
gonad tissue. Eggs were regularly checked for germinal vesicle<br />
breakdown and sperm cells were checked for motility under a<br />
compound microscope. After approximately 12 h, 2 ml of a highly<br />
diluted sperm suspension (testes of three to five adults in approximately<br />
100 ml filtered sea water) were added to the beakers containing<br />
eggs. Developing larvae were fixed at various stages after<br />
fertilization (from 34 h post-fertilization [hpf] to 17 days post-settlement)<br />
in 4% paraformaldehyde in 0.1 M phosphate buffer (PB)<br />
for 90 min. Thereafter, larvae were washed three times for 15 min<br />
each in 0.1 M PB and finally stored in 0.1 M PB containing 0.1%<br />
NaN 3 at 41C.<br />
Immunocytochemistry, confocal laserscanning<br />
microscopy (CLSM), and three-dimensional (3D)<br />
reconstruction<br />
Before staining, larvae were washed thrice for 15 min each in PB<br />
and incubated for 1 h in PB containing 0.2% Triton X-100<br />
(Sigma-Aldrich, St. Louis, MO, USA) at room temperature to<br />
permeabilize the tissue. For F-actin staining, specimens were left<br />
overnight at 41C in0.1M PB containing 0.2% Triton X-100 and<br />
1:40 diluted Alexa Fluor 488 phalloidin (Invitrogen, Molecular<br />
Probes, Eugene, OR, USA). For serotonin and a-tubulin staining,<br />
specimens were first incubated overnight at 41C in 6% normal goat<br />
serum in 0.1 M PB and 0.2% Triton X-100 (blocking solution).<br />
Second, specimens were incubated for 24 h at 41C in blocking solution<br />
containing either a 1:800 diluted polyclonal primary serotonin<br />
antibody (Zymed, Carlton Court, CA, USA), or a 1:500<br />
diluted monoclonal primary acetylated a-tubulin antibody (Sigma-<br />
Aldrich). Third, specimens were washed in the permeabilization<br />
solution overnight at 41C with four changes. Then, the secondary<br />
antibodies (either Alexa Fluor 633-conjugated goat anti-rabbit,<br />
Invitrogen or TRITC-conjugated goat anti-rabbit, Sigma-Aldrich)<br />
were added in a 1:300 dilution to the blocking solution and the<br />
samples were incubated for 24 h. Subsequently, the specimens were<br />
washed three times for 15 min each in 0.1 M PB and embedded in<br />
Fluoromount G (Southern Biotech, Birmingham, AL, USA) on<br />
glass slides. Negative controls omitting either the phalloidin dye or<br />
the respective secondary antibody were performed in order to test<br />
for signal specificity and rendered no signal. The samples were<br />
analyzed with a Leica DM RXE 6 TL fluorescence microscope<br />
equipped with a TCS SP2 AOBS laserscanning device (Leica Microsystems,<br />
Wetzlar, Germany). Animals were scanned with 0.16–<br />
0.49 mm step size, and the resulting image stacks were merged into<br />
maximum projection images. In addition, light micrographs were<br />
recorded to allow overlay with the CLSM images for exact orientation<br />
and localization of the muscle and nervous systems within<br />
the animals. Adobe Photoshop CS3 software (Adobe, San Jose,<br />
CA, USA) was used to create overlay images and for assembling<br />
the figure plates. The sketch drawings were generated with Adobe<br />
Illustrator CS3 (Adobe), and the 3D reconstructions were created<br />
with the Imaris imaging software version 5.7.2 (Bitplane, Zu¨ rich,<br />
Switzerland) based on the CLSM image stacks.<br />
RESULTS<br />
Brachiopod neuromuscular development 17<br />
Myogenesis<br />
The first signals of F-actin were found in the setae pouches of<br />
bilobed larvae at the onset of setae formation. The six setae<br />
pouches are distributed in pairs along the dorsal ridge of the<br />
posterior lobe (Fig. 1A). As the setae grow, the setae pouch<br />
muscles develop further into spherical systems (Figs. 1, B and<br />
G–I and 2A). Later in development, the setae pouch muscles<br />
get interconnected by two bundles of medioventral longitudinal<br />
muscles, which run ventrally from anterior to posterior<br />
(Figs. 1, C and D and G–I and 2A). The medioventral longitudinal<br />
muscle strands get interconnected by transversal<br />
muscles (Fig. 1, B–D and G–I) that are distributed homogenously<br />
in early stages (Fig. 1B) and concentrate into three<br />
bundles in later stages (Fig. 1D). Accordingly, the metamorphic<br />
competent larva has setae pouch muscles, medioventral<br />
longitudinal muscles, and transversal muscles. During metamorphosis,<br />
the larval musculature is replaced by the juvenile<br />
musculature, which most likely develops entirely de novo, that<br />
is, independent of the larval muscle systems (Fig. 1E). The
Chapter III<br />
55<br />
18 EVOLUTION & DEVELOPMENT Vol. 12, No. 1, January--February 2010<br />
Fig. 1. Muscle development in Novocrania anomala. Overlay of maximum projection micrographs from phalloidin staining and light<br />
micrographs. Anterior faces upwards and scale bars equal 50 mm. (A) Larva with anterior lobe (AL), posterior lobe (PL), and early signs of<br />
F-actin in the three pairs of setae pouches (arrows) along the dorsal ridge of the posterior lobe. (B) Larva with setae (se), anterior lobe (AL),<br />
posterior lobe (PL), setae pouch muscles (arrows), homogenously distributed transversal muscles (asterisks), and a distinct F-actin-rich area<br />
(arrowheads), which might be involved in cementing the larva to the substrate during settlement. (C) Later larval stage with setae pouch<br />
muscles (arrows), medioventral longitudinal muscles (empty arrows), and F-actin-rich area (arrowhead) on the dorsal side. (D) Metamorphic<br />
competent larva in ventral view with setae (se) and setae pouch muscles (arrows), which are ventrally interconnected by two strands<br />
of medioventral longitudinal muscles (empty arrows). The medioventral longitudinal muscles are interconnected by transversal muscles,<br />
which at this stage are concentrated into three bundles (asterisks). (E) Specimen during metamorphosis with remnants of larval setae pouch<br />
muscles (arrows) and larval medioventral longitudinal muscles (empty arrows), which are most probably undergoing resorption. The adult<br />
anterior adductor muscles (aad) start to develop. (F) Juvenile with mantle margin muscles (mm), anterior adductor muscle (aad), oblique<br />
muscle (ob), and posterior adductor muscles (pad). (G–I) Three-dimensional reconstruction of the dataset shown in (D). (G) Ventral view of<br />
the musculature of a fully developed larva with medioventral longitudinal muscles (red), setae pouch muscles (yellow), and transversal<br />
muscle (asterisk). (H) Same specimen as in (G), anterior view. (I) Same specimen as in (G), dorsal view.
56 Chapter III<br />
Altenburger and Wanninger<br />
Brachiopod neuromuscular development 19<br />
Fig. 2. Semischematic representation of<br />
the larval musculature of craniiform and<br />
rhynchonelliform brachiopods. (A) Musculature<br />
of Novocrania anomala with<br />
setae pouch muscles (red circles), medioventral<br />
longitudinal muscles (white), and<br />
transversal muscles (yellow-grey). Size of<br />
the specimen is approximately 150 mm.<br />
(B) Musculature of Argyrotheca cordata<br />
based on Altenburger and Wanninger<br />
(2009) with pedicle muscles (beige), longitudinal<br />
muscles (orange), central mantle<br />
muscles (brown), U-shaped muscle<br />
(green), setae pouch muscles (red circles),<br />
circular mantle muscle (light blue), serial<br />
mantle muscles (dark orange), setae muscles<br />
(purple), apical longitudinal muscles<br />
(dark blue), and apical transversal muscle<br />
(yellow). Size of the specimen is approximately<br />
280 mm.<br />
juvenile musculature comprises mantle margin muscles,<br />
oblique muscles, as well as anterior and posterior adductor<br />
muscles (Fig. 1F).<br />
Neurogenesis<br />
The first signals of serotonin-like immunoreactivity appear in<br />
fully developed, metamorphic competent, bilobed larvae at<br />
approximately 86 hpf (Table 1). At this stage, four flaskshaped<br />
cells are present in the anterior-most part of the apical<br />
lobe (Fig. 3, A–D). They are oriented in different directions<br />
with only one pointing toward the apical pole of the larva.<br />
The flask-shaped cells are connected to two ventral neurites<br />
that extend posteriorly (Fig. 3, A–D). The flask-shaped cells<br />
are lost during metamorphosis, and early juveniles have two<br />
ventral neurites that project from the anterior lobe into the<br />
posterior lobe (Fig. 3E). During subsequent development, the<br />
ventral neurites become interconnected by a median commissure<br />
in the mid-part of the juvenile (Fig. 3F).<br />
The axonal marker a-tubulin is first expressed in juveniles 5<br />
days after metamorphosis (Fig. 4A). Two solid neurite bundles<br />
develop ventrolaterally in the anterior lobe of the juvenile and<br />
subsequently grow in posterior direction into the posterior lobe<br />
(Fig. 4B). Later in development, these neurite bundles close by<br />
an anterior and a posterior commissure, and the median commissure<br />
is established (Fig. 4, C–F). Serially arranged mantle<br />
neurites extend from the anterior part of the ventral neurite<br />
bundles in a lateral direction toward the mantle margin of<br />
the juvenile (Fig. 4, B–F). Comparison of the position of the<br />
a-tubulin signal in the juvenile and the serotonin-like signal in<br />
the larva suggests that the larval ventral neurites are the earliest<br />
neurites of the future ventral neurite bundles of the juvenile.<br />
Table 1. Landmarks of Novocrania anomala development at 141C<br />
Age (hours post<br />
fertilization)<br />
Gross morphology<br />
Myoanatomy as inferred by<br />
F-actin staining<br />
3–4 First cleavage No signal No signal<br />
26–30 Swimming, spherical gastrula No signal No signal<br />
42–49 Swimming, elongated gastrula No signal No signal<br />
65–73 Swimming, bilobed larva with<br />
setae starting to develop<br />
First signals of actin in setae<br />
pouches (Fig. 1A)<br />
No signal<br />
86–96 Fully established, swimming,<br />
bilobed larva with long setae<br />
168 Settled juvenile after metamorphosis<br />
Fully developed larval musculature<br />
with setae pouch muscles, longitudinal<br />
muscles, and transversal muscles<br />
(Fig. 1D)<br />
Juvenile with mantle margin muscles,<br />
anterior adductors, and posterior<br />
adductors (Fig. 1F)<br />
Neuroanatomy as inferred by<br />
antibody staining<br />
Larval nervous system with four<br />
flask-shaped cells in the apical organ<br />
and two ventral neurites (Fig.<br />
3, B–D)<br />
Juvenile with two ventral neurite<br />
bundles, commissures, and serially<br />
arranged neurites (Figs. 3, E and<br />
F and 4, A–F)
Chapter III<br />
57<br />
20 EVOLUTION & DEVELOPMENT Vol. 12, No. 1, January--February 2010<br />
Fig. 3. Development of the serotonergic<br />
nervous system in Novocrania anomala.<br />
(A, B, E, and F) Overlay of maximum<br />
projection micrographs of serotonin<br />
staining and light micrographs. (C and<br />
D) Three-dimensional reconstruction of<br />
the dataset shown in B. Anterior faces<br />
upwards and scale bars equal 50 mm. (A<br />
and B) Metamorphic competent larva<br />
with three pairs of setae bundles (se) and<br />
four flask-shaped serotonergic cells (asterisks)<br />
in the anterior part of the apical<br />
lobe (AL), as well as two ventral<br />
serotonergic neurites (arrows) running<br />
from the apical lobe toward the posterior<br />
lobe (PL). The stage in (A) is slightly<br />
younger than that depicted in (B). (C and<br />
D) Same dataset as in (B) with four flaskshaped<br />
serotonergic cells (red) and two<br />
ventral neurites, which are interconnected<br />
anteriorly (yellow). (C) Ventral view. (D)<br />
Lateral view. The flask-shape is visible<br />
only in one cell due to the different position<br />
of the cells. (E) Juvenile during<br />
metamorphosis with two ventral neurites<br />
(arrows), which run from the region of<br />
the former anterior lobe (AL) into the<br />
region of the former posterior lobe (PL).<br />
Larval setae (se) and juvenile shell (s) are<br />
present. (F) Later stage of a juvenile with<br />
two ventral neurites (arrows) which are<br />
interconnected by a median commissure<br />
(mco).<br />
DISCUSSION<br />
Comparative brachiopod myoanatomy<br />
The musculature of fully developed Novocrania anomala larvae<br />
consists of setae pouch muscles, the medioventral longitudinal<br />
muscles that interconnect these setae pouch muscles,<br />
and transversal muscles that interconnect the medioventral<br />
longitudinal muscles (Table 1 and Fig. 2A). This relatively<br />
simple muscular organization differs significantly from that of<br />
articulate brachiopod larvae, which comprises pedicle muscles,<br />
longitudinal muscles, a circular mantle muscle, central<br />
mantle muscles, a U-shaped muscle, serially arranged mantle<br />
muscles, setae muscles, setae pouch muscles, apical longitudinal<br />
muscles, and an apical transversal muscle (Fig. 2B; see<br />
also Altenburger and Wanninger 2009). Unfortunately, very<br />
little is known about brachiopod larval ecology and behavior
58 Chapter III<br />
Altenburger and Wanninger<br />
Brachiopod neuromuscular development 21<br />
Fig. 4. Development of the nervous system<br />
in Novocrania anomala as revealed by<br />
acetylated a-tubulin staining. (A–D)<br />
Overlay of maximum projection micrograph<br />
of a-tubulin staining and light micrograph.<br />
(E and F) Three-dimensional<br />
reconstructions of the dataset shown in<br />
(D). Anterior faces upwards and scale<br />
bars equal 50 mm. (A) First a-tubulin signal<br />
in a juvenile 5 days after metamorphosis.<br />
The former larval apical lobe<br />
(AL) and posterior lobe (PL) are still<br />
visible under the shell (s) of the juvenile.<br />
Two ventral neurite bundles develop in<br />
the anterior lobe (arrows). The juvenile<br />
body is still covered by larval cilia (ci).<br />
Some serially arranged neurites (sn) extend<br />
inwards from the ventral neurite<br />
bundles. (B) The ventral neurite bundles<br />
(arrows) elongate further in posterior direction.<br />
A median commissure (mco)<br />
starts to form. From the anterior portion<br />
of the ventral neurite bundles, serially arranged<br />
mantle neurites (smn) extend distally<br />
outwards, and serially arranged<br />
neurites (sn) extend inwards. The cilia of<br />
the juvenile gut (gu) are visible in the<br />
median region of the juvenile. (C) Juvenile<br />
with the same structures as in (B).<br />
The median commissure (mco) is closed<br />
and the ventral neurite bundles (arrows)<br />
have fused anteriorly to form the anterior<br />
commissure (aco). (D) Neural anatomy<br />
of a juvenile 17 days after metamorphosis<br />
with an anterior commissure (aco), a median<br />
commissure (mco), and a posterior<br />
commissure (pco) that interconnect the<br />
ventral neural bundles (arrows). In addition,<br />
the serially arranged mantle neurites<br />
(smn), which extend toward the edge of<br />
the juvenile mantle, are visible. (E) Threedimensional<br />
reconstruction of the dataset<br />
shown in (D), dorsal view. (F) Three-dimensional<br />
reconstruction of the dataset<br />
shown in (D). Postero-dorsal view demonstrating<br />
that the ventral neurite bundles<br />
(yellow) and the serially arranged<br />
mantle neurites (green) bend ventrally.<br />
(James et al. 1992). Rhynchonelliform larvae show a change<br />
from positive to negative phototactism when reaching metamorphic<br />
competence. In laboratory cultures, they swim in the<br />
culture dish with the anterior lobe or the ventral side of the<br />
body repeatedly forming contact with the bottom of the dish,<br />
probably probing for a suitable place for settlement (Chuang<br />
1996). We observed a similar behavior in Novocrania anomala<br />
larvae before metamorphosis.<br />
At the current state of knowledge, it remains difficult to<br />
relate the differences in larval myoanatomy to aspects concerning<br />
the ecology of the respective brachiopod larvae, because<br />
the latter remains virtually unknown (James et al. 1992).<br />
An earlier study showed that larvae of Novocrania anomala<br />
are able to settle 4 days after fertilization (Nielsen 1991), although<br />
we observed this behavior only in 7-day-old larvae.<br />
Rhynchonelliform brachiopods are known to settle after 3
Chapter III<br />
59<br />
22 EVOLUTION & DEVELOPMENT Vol. 12, No. 1, January--February 2010<br />
(Terebratulina retusa), 5–14 (Terebratalia transversa) (own<br />
observations), or up to 160 days (Liothyrella uva) (see Peck<br />
and Robinson 1994 for listed overview). However, whether or<br />
not these differences in the planktonic lifespan of brachiopod<br />
larvae accounts for the differences in their myoanatomy remains<br />
speculative. Instead, we consider the dissimilarities in<br />
how metamorphosis is achieved in craniiform (inarticulate)<br />
and rhynchonelliform (articulate) larvae as a possible reason<br />
for this morphological variation. During metamorphosis, larvae<br />
of Novocrania anomala curl ventrally by contraction of<br />
the paired medioventral muscles and attach to the substrate<br />
via the epithelium at the posterior end of the larva. The brachial<br />
valve is then secreted by the median part of the dorsal<br />
epithelium and the pedicle valve is secreted by the attachment<br />
epithelium (Nielsen 1991). Larvae of the rhynchonelliform<br />
brachiopod T. transversa attach via a secretory product produced<br />
by the distal tip of the pedicle lobe at the posterior end<br />
of the larva. After attachment, the mantle lobe flips over the<br />
apical lobe and secretes a protegulum containing calcium<br />
carbonate (Stricker and Reed 1985; Freeman 1993).<br />
The phylogenetic relationship of craniiforms to the other<br />
brachiopod subtaxa is still controversial. Based on their lack of<br />
a valve-to-valve articulation they have traditionally been<br />
grouped together with other inarticulated groups (Williams<br />
and Rowell 1965a, b). This view is supported by molecular<br />
analyses based on 18S rDNA sequences, which either place the<br />
craniiforms within the linguliforms (Cohen 2000) or as the<br />
direct sister-group to the linguliforms (Cohen and Weydmann<br />
2005). Other morphological characters such as the presence of<br />
an anus and a lophophore without internal mineralized support<br />
underpins a close relationship of craniiform and linguliform<br />
brachiopods (Carlson 1995). However, based on the<br />
lecithotrophy of the larvae and the presence of a calcareous<br />
shell in the adults, craniiform brachiopods have been proposed<br />
to be closer related to the rhynchonelliforms rather than to<br />
the linguliforms, which have a free-swimming planktotrophic<br />
life cycle stage that closely resembles the morphology of juvenile<br />
brachiopods (Nielsen 1991). An alternative scenario proposes<br />
that lecithotrophic larvae equipped with larval setae are<br />
basal for Brachiopoda and that the swimming ‘‘paralarvae’’ of<br />
lingulids constitute a planktonic juvenile stage, thereby implying<br />
that the linguliforms have secondarily lost the lecithotrophic<br />
larva (Lu¨ ter 2001). Our data corroborates this view.<br />
The musculature of postmetamorphosic Novocrania anomala<br />
comprises anterior adductors, posterior adductors, and<br />
oblique lateral muscles. This corresponds to the musculature<br />
found in adults, which in addition have brachial protractor<br />
muscles at the base of the lophophore, an unpaired median<br />
muscle, and oblique internal muscles (Bulman 1939; Helmcke<br />
1939; Williams and Rowell 1965a, b). In the present study we<br />
found mantle retractor muscles, which had previously been<br />
undescribed for Novocrania anomala and which correspond to<br />
the respective muscles found in the rhynchonelliform brachiopods<br />
Argyrotheca cordata, Argyrotheca cistellula, and<br />
Terebratalia transversa (Altenburger and Wanninger 2009).<br />
Given the distinct differences in the larval musculature of<br />
craniiforms and rhynchonelliforms, it is difficult to infer a<br />
muscular ground pattern for brachiopod larvae. However, it<br />
appears likely that a hypothetical ancestral brachiopod larva<br />
had at least setae pouch muscles and a musculature that interconnect<br />
these setae pouch muscles.<br />
Neurogenesis<br />
The serotonergic nervous system of Novocrania anomala starts<br />
to develop in fully established larvae (see Table 1), and shows<br />
an apical organ consisting of four flask-shaped cells and two<br />
lateroventral neurites, which grow from the anterior lobe into<br />
the posterior lobe. These results constitute the first unambiguous<br />
account of the presence of an apical organ with<br />
serotonergic flask-shaped cells in a lecithotrophic brachiopod<br />
larva. Similar apical organs containing flask-shaped cells have<br />
been found in a wide range of lophotrochozoans including<br />
entoprocts (Wanninger et al. 2007), mollusks (Voronezhskaya<br />
et al. 2002; Wanninger and Haszprunar 2003), annelids<br />
(Voronezhskaya et al. 2003), and ectoprocts (Pires and Woollacott<br />
1997; Shimizu et al. 2000). The finding of an apical<br />
organ with serotonergic flask-shaped cells in a lecithotrophic<br />
brachiopod larva suggests that such an apical organ was also<br />
present in the larva of the last common lophotrochozoan ancestor<br />
(Wanninger 2009). Interestingly, such flask cells are<br />
also present in larvae of the demosponge Amphimedon queenslandica,<br />
but whether or not they express serotonin-like<br />
immunoreactivity in this species remains unknown (Sakarya<br />
et al. 2007). Accordingly, it appears that the evolution of<br />
flask-shaped cells in metazoan larvae predated the poriferan–<br />
eumetazoan split, whereby it remains possible that these cells<br />
only acquired serotonin-like immunoreactivity in the lophotrochozoan<br />
lineage. In case of such a scenario, serotoninexpressing<br />
flask cells would be a distinct apomorphy for the<br />
entire Lophotrochozoa.<br />
Similar to the vast majority of lophotrochozoan larvae,<br />
but significantly different to the situation found in the entoproct<br />
creeping-type larva and the larva of polyplacophoran<br />
mollusks, the apical organ of Novocrania anomala is comparatively<br />
simple, thus supporting the notion that a simple apical<br />
organ was present in the ‘‘ur-lophotrochozoan’’ larva,<br />
whereas a complex apical organ is likely to be a synapomorphy<br />
of a monophyletic Entoprocta1Mollusca (Tetraneuralia<br />
concept; see Wanninger 2009).<br />
A serotonergic nervous system has been described previously<br />
for planktotrophic linguliform brachiopod ‘‘paralarvae.’’<br />
There, the apical organ is located at the base of the<br />
median tentacle and comprises numerous serotonergic cells<br />
(Hay-Schmidt 1992). Although it is tempting to speculate that<br />
this neural structure might correspond to the spiralian-type
60 Chapter III<br />
Altenburger and Wanninger<br />
apical organ described herein for Novocrania anomala, it is<br />
important to note (i) that a flask-shaped character could not<br />
be assigned to the apical organ cells of these linguliform paralarvae<br />
and (ii) that the number of cells in their apical organ<br />
is considerably higher than that of the other spiralian larvae.<br />
Overall, the ‘‘apical organ’’ of linguliform larvae resembles<br />
more closely the one found in phoronid and deuterostome<br />
larvae (Santagata 2002), the homology of which remains to be<br />
proven. The suggested derived character of the nervous system<br />
of linguliform brachiopod paralarvae is consistent with<br />
the view that linguliforms have lost the lecithotrophic larva<br />
and have secondarily acquired a planktotrophic life cycle<br />
stage via a stage that resembles a swimming juvenile rather<br />
than a ‘‘true’’ brachiopod larva (Lüter 2001).<br />
We found a-tubulin-positive neural tissue solely in postmetamorphic<br />
specimens of Novocrania anomala. The a-tubulin<br />
signal is located in the same region as the serotonin-like<br />
signal and shows two ventral neurite bundles that are interconnected<br />
by one commissure at the anterior end, one at the<br />
posterior end, and by a median commissure. The fact that we<br />
did not find a-tubulin in the Novocrania anomala larvae that<br />
exhibit serotonergic neurites demonstrates that tubulin<br />
alone is not a reliable marker for nervous structures in<br />
lophotrochozoan larvae. The tubulinergic nervous system in<br />
juvenile Novocrania anomala outlines the adult nervous<br />
system, which consists of two ventral neurite bundles, a subesophageal<br />
and a supraesophageal commissure, and mediodorsal<br />
mantle neurites (Blochmann 1892; Bullock and<br />
Horridge 1965). The anterior ventral neurite bundles form<br />
the arm neurites of the lophophore. Perpendicular from these<br />
arm neurites extend accessory brachial neurites (Williams and<br />
Rowell 1965a, b).<br />
Despite some classical studies, the adult neural anatomy of<br />
brachiopods is only poorly known (James et al. 1992). In the<br />
articulate Gryphus vitreus the nervous system comprises a<br />
transverse supraenteric ganglion and a subenteric ganglion<br />
lying above and below the esophagus, as does the subesophageal<br />
and supraesophageal commissure in Novocrania<br />
anomala (Bullock and Horridge 1965). Our study provides a<br />
first step toward an understanding of the larval anatomy,<br />
neurotransmitter distribution, and development of the nervous<br />
system in brachiopod taxa with lecithotrophic larvae.<br />
Although additional data are needed to assess the brachiopod<br />
neural ground pattern, the finding that serotonergic flaskshaped<br />
cells similar to those found in spiralian larvae do occur<br />
in the apical organ of Novocrania anomala larvae strengthens<br />
the hypo<strong>thesis</strong> that this cell type was also present in the last<br />
common ancestor of Lophotrochozoa (see Wanninger 2009).<br />
Acknowledgments<br />
We are grateful to Matthias Obst and the staff of the Sven Lovén<br />
Centre for Marine Science, Kristineberg, Sweden for help with collection<br />
of adult animals and for providing laboratory space. This<br />
study was funded by the Danish Agency for Science, Technology and<br />
Innovation (grant no. 645-06-0294 to A. W.). Research in the laboratory<br />
of A. W. is further supported by the EU-funded Marie Curie<br />
Network MOLMORPH (contract grant no. MEST-CT-2005-020542).<br />
REFERENCES<br />
Brachiopod neuromuscular development 23<br />
Altenburger, A., and Wanninger, A. 2009. Comparative larval myogenesis<br />
and adult myoanatomy of the rhynchonelliform (articulate) brachiopods<br />
Argyrotheca cordata, A. cistellula, and Terebratalia transversa. Front.<br />
Zool. 6: 3.<br />
Atkins, D., and Rudwick, M. J. S. 1962. The lophophore and ciliary feeding<br />
mechanisms of the brachiopod Crania anomala (Mu¨ ller). J. Mar. Biol.<br />
Assoc. UK 42: 469–480.<br />
Blochmann, F. 1892. Untersuchungen u¨ber den Bau der Brachiopoden. Gustav<br />
Fischer, Jena.<br />
Bulman, O. M. B. 1939. Muscle systems of some inarticulate brachiopods.<br />
Geol. Mag. 76: 434–444.<br />
Bullock, T. H., and Horridge, G. A. 1965. Lophophorate phyla: Ectoprocta,<br />
Brachiopoda, and Phoronida. In Structure and Function in the<br />
Nervous System of Invertebrates. W.H.Freeman,NewYork,pp.631–<br />
647.<br />
Carlson, S. J. 1995. Phylogenetic relationships among extant brachiopods.<br />
Cladistics 11: 131–197.<br />
Cohen, B. L. 2000. Monophyly of brachiopods and phoronids: reconciliation<br />
of molecular evidence with Linnaean classification (the subphylum<br />
Phoroniformea nov.). Proc. R. Soc. B 267: 225–231.<br />
Cohen, B. L., Long, S. L., and Saito, M. 2008. Living craniids: preliminary<br />
molecular evidence of their inter-relationships. Fossils Strata 54: 283–287.<br />
Cohen, B. L., and Weydmann, A. 2005. Molecular evidence that phoronids<br />
are a subtaxon of brachiopods (Brachiopoda: Phoronata) and that genetic<br />
divergence of metazoan phyla began long before the early Cambrian.<br />
Org. Divers. Evol. 5: 253–273.<br />
Chuang, S. H. 1996. Competence, pre- and post-settlement choices of articulate<br />
brachiopod larvae. In P. Copper and J. Jin (eds.). Brachiopods:<br />
Proceedings of the Third International Brachiopod Congress. A.A.Balkema,<br />
Rotterdam, pp. 65–67.<br />
Freeman, G. 1993. Metamorphosis in the brachiopod TerebrataliaFevidence<br />
for a role of calcium-channel function and the dissociation of shell<br />
formation from settlement. Biol. Bull. 184: 15–24.<br />
Freeman, G. 2000. Regional specification during embryogenesis in the<br />
craniiform brachiopod Crania anomala. Dev. Biol. 227: 219–238.<br />
Freeman, G., and Lundelius, J. W. 1999. Changes in the timing of mantle<br />
formation and larval life history traits in linguliform and craniiform<br />
brachiopods. Lethaia 32: 197–216.<br />
Gee, H. 1995. Lophophorates prove likewise variable. Nature 374: 493.<br />
Halanych, K. M. 2004. The new view of animal phylogeny. Annu. Rev. Ecol.<br />
Evol. Syst. 35: 229–256.<br />
Hay-Schmidt, A. 1992. Ultrastructure and immunocytochemistry of the<br />
nervous system of the larvae of Lingula anatina and Glottidia sp. (Brachiopoda).<br />
Zoomorphology 112: 189–205.<br />
Hay-Schmidt, A. 2000. The evolution of the serotonergic nervous system.<br />
Proc. R. Soc. B 267: 1071–1079.<br />
Hejnol, A., et al. 2009. Assessing the root of bilaterian animals with scalable<br />
phylogenomic methods. Proc. R. Soc. B 276: 4261–4270.<br />
Helmcke, J. G. 1939. Die Muskeln der Brachiopoden. Zool. Jahrb. Abt.<br />
Syst. Oekol. Geogr. Tiere 72: 100–140.<br />
Holmer, L. E. 2001. Phylogeny and classification: Linguliformea and<br />
Craniiformea. Paleontol. Soc. Paper 7: 11–26.<br />
James, M. A., Ansell, A. D., Collins, M. J., Curry, G. B., Peck, L. S., and<br />
Rhodes, M. C. 1992. Biology of living brachiopods. Adv. Mar. Biol. 28:<br />
175–387.<br />
Lee, D. E., and Brunton, C. H. C. 1986. Neocrania n. gen., and a revision of<br />
Cretaceous–Recent brachiopod genera in the family Craniidae. Bull. Nat.<br />
Hist. Mus. London (Geol.) 40: 141–160.<br />
Lee, D. E., and Brunton, C. H. C. 2001. Novocrania, anewnameforthe<br />
genus Neocrania Lee & Brunton, 1986 (Brachiopoda, Craniida), preoc-
Chapter III<br />
61<br />
24 EVOLUTION & DEVELOPMENT Vol. 12, No. 1, January--February 2010<br />
cupied by Neocrania Davis, 1978 (Insecta, Lepidoptera). Bull. Nat. Hist.<br />
Mus. London (Geol.) 57: 5.<br />
Lu¨ ter, C. 2001. Brachiopod larval setaeFa key to the phylum’s ancestral<br />
life cycle? In C. H. C. Brunton, L. R. M. Cocks, and S. L. Long (eds.).<br />
Brachiopods Past and Present. Taylor & Francis, London, pp. 46–55.<br />
Nielsen, C. 1991. The development of the brachiopod Crania (Neocrania)<br />
anomala (O. F. Mu¨ ller) and its phylogenetic significance. Acta Zool. 72:<br />
7–28.<br />
Nielsen, C. 2002. The phylogenetic position of Entoprocta, Ectoprocta,<br />
Phoronida, and Brachiopoda. Integr. Comp. Biol. 42: 685–691.<br />
Paps, J., Bagun˜ a` , J., and Riutort, M. 2009. Lophotrochozoa internal phylogeny:<br />
new insights from an up-to-date analysis of nuclear ribosomal<br />
genes. Proc. R. Soc. B 276: 1245–1254.<br />
Peck, L. S., and Robinson, K. 1994. Pelagic larval development in<br />
the brooding Antarctic brachiopod Liothyrella uva. Mar. Biol. 120: 279–<br />
286.<br />
Pires, A., and Woollacott, R. M. 1997. Serotonin and dopamine have opposite<br />
effects on phototaxis in larvae of the bryozoan Bugula neritina.<br />
Biol. Bull. 192: 399–409.<br />
Rowell, A. J. 1960. Some early stages in the development of the brachiopod<br />
Crania anomala (Mu¨ ller). Ann. Mag. Nat. Hist. 13: 35–56.<br />
Sakarya, O., et al. 2007. A post-synaptic scaffold at the origin of the animal<br />
kingdom. PLoS One 2: e506.<br />
Santagata, S. 2002. Structure and metamorphic remodeling of the larval<br />
nervous system and musculature of Phoronis pallida (Phoronida). Evol.<br />
Dev. 4: 28–42.<br />
Shimizu, K., Hunter, E., and Fusetani, N. 2000. Localisation of biogenic<br />
amines in larvae of Bugula neritina (Bryozoa: Cheilostomatida) and their<br />
effects on settlement. Mar. Biol. 136: 1–9.<br />
Stricker, S. A., and Reed, C. G. 1985. The ontogeny of shell secretion in<br />
Terebratalia transversa (Brachiopoda, Articulata). 1. Development of the<br />
mantle. J. Morphol. 183: 233–250.<br />
Voronezhskaya, E. E., Tsitrin, E. B., and Nezlin, L. P. 2003. Neuronal<br />
development in larval polychaete Phyllodoce maculata (Phyllodocidae).<br />
J. Comp. Neurol. 455: 299–309.<br />
Voronezhskaya, E. E., Tyurin, S. A., and Nezlin, L. P. 2002. Neuronal<br />
development in larval chiton Ischnochiton hakodadensis (Mollusca: Polyplacophora).<br />
J. Comp. Neurol. 444: 25–38.<br />
Wanninger, A. 2009. Shaping the things to come: ontogeny of Lophotrochozoan<br />
neuromuscular systems and the Tetraneuralia concept. Biol.<br />
Bull. 216: 293–306.<br />
Wanninger, A., Fuchs, J., and Haszprunar, G. 2007. Anatomy of the<br />
serotonergic nervous system of an entoproct creeping-type larva and its<br />
phylogenetic implications. Invertebr. Biol. 126: 268–278.<br />
Wanninger, A., and Haszprunar, G. 2003. The development of the<br />
serotonergic and FMRF-amidergic nervous system in Antalis entalis<br />
(Mollusca, Scaphopoda). Zoomorphology 122: 77–85.<br />
Williams, A., Carlson, S. J., Brunton, C. H. C., Holmer, L. E., and Popov,<br />
L. 1996. A supra-ordinal classification of the Brachiopoda. Proc. R. Soc.<br />
B 351: 1171–1193.<br />
Williams, A., and Rowell, A. J. 1965a. Brachiopod anatomy. In R. C.<br />
Moore (ed.). Treatise on Invertebrate Paleontology, Part H, Brachiopoda.<br />
Geological Society of America and University of Kansas Press, Lawrence,<br />
KS, pp. 6–57.<br />
Williams, A., and Rowell, A. J. 1965b. Evolution and phylogeny. In R. C.<br />
Moore (ed.). Treatise on Invertebrate Paleontology, Part H, Brachiopoda.<br />
The Geological Society of America and The University of Kansas Press,<br />
Lawrence, KS, pp. 164–199.
62 Chapter IV<br />
Chapter IV<br />
Altenburger, A., Martinez, P. & Wanninger, A. First expression<br />
study of homeobox genes in Brachiopoda: the role of Not and Cdx<br />
in bodyplan patterning and germ layer specification. Submitted
Chapter IV<br />
Submitted manuscript<br />
63<br />
First expression study of homeobox genes<br />
in Brachiopoda: the role of Not and Cdx in<br />
bodyplan patterning, neurogenesis, and germ<br />
layer specification<br />
Andreas Altenburger 1 , Pedro Martinez 2, 3, Andreas Wanninger 1*<br />
1<br />
University of Copenhagen, Department of Biology, Research Group for Comparative Zoology,<br />
Universitetsparken 15, DK-2100 Copenhagen Ø, Denmark<br />
2<br />
Universitat de Barcelona, Facultat de Biología, Departament de Genètica, Av. Diagonal 645,<br />
ES-08028 Barcelona, Spain<br />
3<br />
Institució Catalana de Recerca i Estudis Avançats (ICREA)<br />
*corresponding author. E-mail: awanninger@bio.ku.dk<br />
ABSTRACT<br />
Not is a homeobox containing gene<br />
that regulates the formation of the<br />
notochord in chordates, while Caudal<br />
(Cdx) is a ParaHox gene involved in<br />
the formation of posterior tissues of<br />
various animal phyla. Here, we present<br />
the first expression data of a Not<br />
and a Cdx homolog in the articulate<br />
brachiopod Terebratalia transversa.<br />
The T. transversa homolog, TtrNot,<br />
is expressed in the ectoderm from<br />
the beginning of gastrulation until<br />
completion of larval development,<br />
which is marked by a three-lobed body<br />
with larval setae. Expression starts at<br />
gastrulation in two areas lateral to the<br />
blastopore and subsequently extends<br />
over the animal pole of the gastrula.<br />
With elongation of the gastrula,<br />
expression at the animal pole narrows<br />
to a small band, whereas the areas<br />
lateral to the blastopore shift slightly<br />
towards the future anterior region of<br />
the larva. Upon formation of the three<br />
larval body lobes, TtrNot expressing<br />
cells are present only in the posterior<br />
part of the apical lobe. Expression<br />
ceases entirely at the onset of larval<br />
setae formation. TtrNot expression<br />
is absent in unfertilized eggs, in<br />
INTRODUCTION<br />
Homeobox genes are characterized<br />
by the presence of a short, wellconserved<br />
DNA fragment, which<br />
encodes for the homeodomain. The<br />
latter is a protein motif of 60 to 63<br />
amino acids, which was first described<br />
embryos prior to gastrulation, and in<br />
settled individuals during and after<br />
metamorphosis. Comparison with<br />
the expression patterns of Not genes<br />
in other metazoan phyla suggests<br />
an ancestral role in gastrulation and<br />
germ layer (ectoderm) specification<br />
with co-opted functions in notochord<br />
formation in chordates and left/right<br />
determination in ambulacrarians and<br />
vertebrates. TtrCdx is first expressed<br />
after gastrulation in the ectoderm of the<br />
gastrula in the posterior region of the<br />
blastopore. Its expression stays stable<br />
in the ectoderm at the posterior pole<br />
of the blastopore until the blastopore<br />
is closed. Thereafter, the expression<br />
remains in the ventral portion of the<br />
mantle lobe of the fully developed larva.<br />
No TtrCdx expression is detectable in<br />
the juvenile after metamorphosis. The<br />
expression of TtrCdx is congruent with<br />
findings in other metazoans, were<br />
genes belonging to the Cdx/caudal<br />
family are predominantly localized<br />
posteriorly during gastrulation and<br />
subsequently play a role in the<br />
formation of posterior tissues.<br />
for Drosophila melanogaster homeotic<br />
genes, and subsequently was found<br />
in all animal phyla studied to date<br />
(McGinnis et al. 1984, Scott and<br />
Weiner 1984, Lanfear and Bromham<br />
2008). Homeobox genes function as<br />
developmental control genes that
64 Submitted manuscript<br />
Chapter IV<br />
encode transcription factors which<br />
activate gene cascades (Hueber and<br />
Lohmann 2008). In the case of the<br />
Not gene, which plays an important<br />
role during notochord formation in<br />
vertebrates (Stein and Kessel 1995,<br />
Talbot et al. 1995, Gont et al. 1996, Stein<br />
et al. 1996, Abdelkhalek et al. 2004),<br />
the downstream genes are known to<br />
regulate mesoderm formation in sea<br />
urchins as well as left/right patterning,<br />
notochord, mesoderm, and somite<br />
formation in vertebrates (Peterson et<br />
al. 1999, Yasuo and Lemaire 2001,<br />
Beckers et al. 2007). Homologs of<br />
the homeobox gene Not have been,<br />
among others, identified in Xenopus<br />
(Xnot), chick (Gnot1, Gnot2), zebrafish<br />
(flh), mouse (noto), Hydra (HvuNot),<br />
Drosophila (90Bre), and the basal<br />
eumetazoan Trichoplax adhaerens<br />
(TadNot), but the developmental<br />
role of Not in invertebrates without a<br />
notochord is largely unknown (Dessain<br />
and McGinnis 1993, von Dassow et al.<br />
1993, Knezevic et al. 1995, Odenthal<br />
et al. 1996, Gauchat et al. 2000,<br />
Martinelli and Spring 2004, Hoskins<br />
et al. 2007). A Not homolog seems<br />
to be lacking in the model sponge<br />
Amphimedon queenslandica (Bernard<br />
Degnan, personal communication).<br />
However, the presence of a Not gene<br />
in cnidarians and Trichoplax indicates<br />
that it was present prior to the evolution<br />
of the mesoderm, and thus long<br />
before the evolution of the notochord.<br />
Accordingly, the ancestral role of Not<br />
remains elusive. Given its confirmed<br />
absence in the poriferan genome<br />
would make it a good candidate for a<br />
eumetazoan apomorphy.<br />
Cdx is a member of the ParaHox gene<br />
cluster which probably originated by<br />
duplication from an ancestral ProtoHox<br />
gene cluster which led to the Hox and<br />
ParaHox clusters, respectively (Brooke<br />
et al. 1998). Cdx has been found to be<br />
involved in the development of posterior<br />
tissues of almost all animal phyla in<br />
which it has been investigated and is<br />
thus often termed “caudal” (Epstein et<br />
al. 1997, Copf et al. 2004). In addition<br />
to the posterior tissues, it was found<br />
to be expressed in the mesoderm of<br />
taxa as diverse as Artemia, Capitella,<br />
Patella, Branchiostoma, and Mus;<br />
in the gut of Drosophila, Capitella,<br />
Branchiostoma, and Mus; and in the<br />
central nervous system of Capitella,<br />
Branchiostoma, and Mus (Macdonald<br />
and Struhl 1986, Duprey et al. 1988,<br />
Le Gouar et al. 2003, Copf et al.<br />
2004, Fröbius and Seaver 2006). Cdx<br />
is absent in the recently sequenced<br />
poriferan Amphimedon queenslandica<br />
(Larroux et al. 2008, Srivastava et<br />
al. 2010), but a gene related to Cdx<br />
is present in Nematostella vectensis,<br />
a representative of Cnidaria, the<br />
proposed sister group to Bilateria<br />
(Chourrout et al. 2006, Quiquand et<br />
al. 2009).<br />
The phylogenetic position of<br />
Brachiopoda within Bilateria is still<br />
controversial (Williams and Carlson<br />
2007, Hejnol et al. 2009, Paps et al.<br />
2009). Most authors include them within<br />
Lophotrochozoa, but their position<br />
within this clade remains unresolved,<br />
and some authors consider them a<br />
sister group to Deuterostomia (Nielsen<br />
2002). Within the phylum, Brachiopoda<br />
comprises three clades: Linguliformea,<br />
Craniiformea, and Rhynchonelliformea.<br />
Linguliformea and Craniiformea are<br />
often considered sister groups and<br />
were traditionally termed “inarticulate”,
Chapter IV<br />
Submitted manuscript<br />
65<br />
because their valves are not connected<br />
to each other by a hinge (Cohen and<br />
Weydmann 2005). We investigated the<br />
brachiopod Terebratalia transversa, a<br />
representative of the rhynchonelliform<br />
(articulate) brachiopods, the largest<br />
group within recent Brachiopoda. So<br />
far, several Hox gene sequences have<br />
been characterized for the linguliform<br />
brachiopod Lingula anatina (de Rosa et<br />
al. 1999). However, no expression data<br />
for any Hox or homeobox containing<br />
genes are currently available for<br />
Brachiopoda. With the investigation of<br />
Not and Cdx expression in Terebratalia<br />
transversa we aim to shed light on the<br />
function of these genes in invertebrate<br />
body patterning and thereby contribute<br />
to the discussion concerning their<br />
ancestral roles in eumetazoan (i.e.,<br />
placozoan, diploblast, and triploblast)<br />
development and evolution.<br />
MATERIAL AND METHODS<br />
Animal collection, rearing, and<br />
fixation<br />
Adult animals were dredged in the vicinity<br />
of the Friday Harbor Laboratories,<br />
Washington, USA, at 48º32’869 N;<br />
122º58’452 W during summer 2008<br />
and spring 2009. The animals were<br />
placed in running seawater tables<br />
at ambient seawater temperature<br />
(approx. 11.5ºC). Embryos were<br />
obtained by artificial fertilization. To<br />
this end, gonads were dissected from<br />
the specimens and stored individually<br />
in beaker glasses. The eggs were<br />
washed several times with seawater<br />
and left in 100ml seawater until<br />
germinal vesicle breakdown, which<br />
usually occurred within 10-16 hours<br />
after dissection. Sperm cells were left<br />
until they had acquired a high degree<br />
of motility, which usually occurred after<br />
4-14 hours. Sperm remained active<br />
until up to 48 hours after dissection.<br />
For fertilization, a few drops of the<br />
sperm suspension were added to the<br />
beaker glasses containing the eggs.<br />
Development of embryos and larvae<br />
was monitored closely and the beaker<br />
glasses were cleaned daily from debris<br />
with help of a glass pipette driven by a<br />
peristaltic pump. Larvae were fixed at<br />
various developmental stages in 4%<br />
paraformaldehyde in 0.5M NaCl, 0.1M<br />
MOPS (pH 7.5) for 8-10 hours at 4ºC,<br />
washed in 50% EtOH for 30 min, and<br />
finally stored in 80% EtOH at -20ºC.<br />
Cloning and in situ hybridization<br />
RNA was extracted from larvae<br />
at various developmental stages<br />
with a miRCURY RNA Isolation Kit<br />
(Exiqon, Vedbaek, Denmark). It was<br />
reversely transcribed into cDNA with<br />
a RETROscript Kit using oligo(dT)<br />
primers (Applied Biosystems/Ambion,<br />
Austin, TX, USA). In order to screen<br />
for homeobox containing genes, the<br />
cDNA was used as template for PCR<br />
reactions with the following degenerate<br />
primers: HoxF 5’-GCT CTA GAR YTN<br />
GAR AAR GAR TT-3’, which recognizes<br />
the peptide sequence ELEKEF, and<br />
HoxR 5’-GGA ATT CRT TYT GRA<br />
ACC ADA TYT T-3’, which recognizes<br />
the peptide sequence KIWFQN<br />
(Murtha et al. 1991; Balavoine and<br />
Telford 1995). PCR was carried out<br />
under the following conditions: 3 min<br />
94 °C, followed by 40 cycles of 45s at<br />
94 °C, 45s at 50 °C, and 60s at 72 °C,<br />
followed by a final extension step of<br />
10 min at 72 °C. PCR products were<br />
purified over column with a QIAquick<br />
Gel Extraction Kit (Qiagen, Venlo, The
66 Submitted manuscript<br />
Chapter IV<br />
Netherlands) and subsequently ligated<br />
into a pGEM-T Easy vector (Promega,<br />
Madison, WI, USA). Ligation products<br />
were transformed into One Shot<br />
TOP10 E. coli competent cells<br />
(Invitrogen, Carlsbad, CA, USA). Cells<br />
were allowed to grow over night; clone<br />
DNA was isolated using a QIAprep<br />
Spin Miniprep Kit (Qiagen). Insert<br />
sequences were sequenced at the<br />
sequencing facility of the University<br />
of Barcelona and identified using<br />
the tBLASTx algorithm. Two specific<br />
forward primers were subsequently<br />
designed from the TtrNot and the<br />
TtrCdx PCR sequences: TtrNotF1 5’-<br />
GGA GAA GGA GTT CGA AAG GCA<br />
ACA A-3’, TtrNotF2 5’-CCG AAT CCC<br />
AAG TGA AGA TCT GGT-3’, TtrCdxF1<br />
5’-CCT GGA GCT GGA GAA GGA<br />
GTT CTG T-3’, and TtrCdxF2 5’-AAC<br />
AAC CTT GTA CTT TCA GAG AGA<br />
CAG G-3’. The specific primers were<br />
used nested in a 3’RACE-PCR using<br />
a SMART RACE kit following the<br />
manufacturer’s protocol (Clontech,<br />
Mountain View, CA, USA). The<br />
sequences of the RACE-PCR products<br />
were again checked by BLAST and the<br />
positive clones were used for in situ<br />
probe production using the DIG RNA<br />
Labeling Kit (SP6/T7, Roche, Basel,<br />
Switzerland).<br />
In situs were done following a standard<br />
protocol with a 5 min proteinase K step<br />
and at least 48 hours of hybridization<br />
time at 40ºC or 45ºC (Martindale<br />
et al. 2004; Hejnol and Martindale<br />
2008). For cohorts aged 0-64 hours<br />
after fertilization (hpf), in situs were<br />
performed on developmental stages<br />
that were 2-4 hours apart, for the age<br />
group of 64-154 hpf, in situs were<br />
done every 5-10 hours, while for later<br />
stages longer intervals were chosen.<br />
The latest stages investigated were<br />
540 hpf old, which corresponded to<br />
420 hours after settlement/onset of<br />
metamorphosis (hps). Sense probes<br />
were generated as controls for in situ<br />
hybridization. Since they didn’t give<br />
any signal they are omitted in the<br />
figures.<br />
Stained specimens were photographed<br />
with a Leica ProgRes C3 digital<br />
camera mounted on a Leica MZ<br />
16F stereomicroscope. Schematic<br />
illustrations were generated using<br />
Adobe Illustrator CS3 and CS4<br />
graphics software (Adobe, San Jose,<br />
CA, USA). Analysis of gene sequences<br />
and primer design was done with CLC<br />
Main Workbench 5 (CLC bio, Aarhus,<br />
Denmark).<br />
Immunostaining and confocal<br />
laserscanning microscopy<br />
Larvae were stained with antibodies<br />
against serotonin (ImmunoStar,<br />
Hudson, WI, USA) and acetylated<br />
α-tubulin (Sigma-Aldrich, St. Louis,<br />
MO, USA). In addition, cell nuclei<br />
were labeled using DAPI (Invitrogen,<br />
Eugene, OR, USA). Prior to staining,<br />
larvae were washed thrice for 15min<br />
each in phosphate buffer (PB) and<br />
incubated for 1h in PB containing<br />
0.2% Triton X-100 (Sigma-Aldrich)<br />
at room temperature. Thereafter,<br />
the larvae were incubated over night<br />
at 4ºC in 6% normal goat serum<br />
in 0.1M PB and 0.2% Triton X-100<br />
(blocking solution). Then, the larvae<br />
were incubated for 24 hours at 4ºC in<br />
blocking solution containing a 1:800<br />
dilution of the polyclonal serotonin<br />
antibody, 3µg/ml DAPI, and a 1:800<br />
dilution of the monoclonal acetylated
Chapter IV<br />
Submitted manuscript<br />
67<br />
Fig. 1 Characterization of the Not sequence of Terebratalia transversa. (A) Not<br />
homeodomain sequence alignment. The accession numbers for the EMBL/GenBank databases<br />
are given in brackets: Terebratalia transversa (Ttr, brachiopod, XXXXXXX), Nematostella<br />
vectensis (Nve, cnidarian, XP_001641364.1), Hydra vulgaris (Hvu, cnidarian, CAB88387.1),<br />
Trichoplax adhaerens (Tad, placozoan, AAQ82694.1), Drosophila melanogaster (Dme, fruit fly,<br />
NP_650701.1), Strongylocentrotus purpuratus (Spu, sea urchin, AAD20328.1), Hemicentrotus<br />
pulcherrimus (Hpu, sea urchin, BAD91047.1), Branchiostoma floridae (Bfl, Florida lancelet,<br />
XP_002601133.1), Danio rerio (Dre, zebrafish, NP_571130.1), Xenopus laevis (X, frog,<br />
NP_001081625.1). The following alternative species and Hox protein sequences were chosen<br />
as outgroups: Drosophila virilis, Antennapedia (DviAnt, fruit fly, AAQ67266.1), Drosophila<br />
melanogaster, Proboscipedia (DmePb, fruit fly, CAA45272), Neanthes virens, Hox7 and<br />
Engrailed (NviHox7 and NviEng, annelid, DQ366682 and DQ366680). Dots represent amino<br />
acid identity with the amino acid sequence of the T. transversa Not protein shown at the top of<br />
the alignment. (B) Alignment tree based on the 52 amino acid sequences shaded in Fig. 1A.<br />
Algorithm = UPGMA; Bootstrap = 10.000 replicates. Bootstrap values are given for each node.<br />
Due to the small number of residues for the analysis, the phylogenetic signal of the tree is<br />
limited. The tree shows, however, that TtrNot clusters with all other Not protein sequences and<br />
thus is a true Not protein.<br />
α-tubulin antibody. Subsequently,<br />
the larvae were washed four times<br />
over a period of 12h in PB containing<br />
0.2% Triton X-100 and an Alexa Fluor<br />
633-conjugated goat anti-rabbit as<br />
well as an Alexa Fluor 488-conjugated<br />
goat anti-mouse secondary antibody<br />
(Invitrogen) in a dilution of 1:400<br />
for 24h at 4ºC. Finally, the larvae<br />
were washed tree times for 15 min<br />
each in 0.1M PB and embedded in<br />
Flouromount G (Southern Biotech,<br />
Birmingham, AL, USA) on glass<br />
slides. The samples were analyzed<br />
with a Leica TCS SP5 II confocal<br />
system (Leica Microsystems, Wetzlar,<br />
Germany). The resulting image stacks<br />
were merged into maximum projection<br />
images and assembled using Adobe<br />
Photoshop CS3 software (Adobe, San<br />
Jose, CA, USA).
68 Submitted manuscript<br />
Chapter IV<br />
Fig. 2 Characterisation of the Cdx sequence of Terebratalia transversa. (A) Cdx<br />
homeodomain sequence alignment. The accession numbers for the EMBL/GenBank databases<br />
are given in brackets: Terebratalia transversa (Ttr, brachiopod, XXXXXXX), Nematostella<br />
vectensis (Nve, cnidarian, DQ500749), Patella vulgata (Pvu, gastropod, AJ518062.1), Capitella<br />
teleta (Cte, annelid, AAZ95508.1), Drosophila melanogaster (Dme, fruit fly, NM_057606.4),<br />
Tribolium castaneum (Tca, flour beetle, NM_001039409.1), Ciona intestinalis (Cin, tunicate,<br />
NP_001071669), Saccoglossus kowalevskii (Sko, hemichordate, NP_001158415), and Mus<br />
musculus (Mmu, mouse, NM_009880.3). The following alternative species and Hox protein<br />
sequences were chosen as outgroups: Drosophila simulans, Abdominal B (DsiAbdB, fruit<br />
fly, XP_002103136), Drosophila melanogaster, Proboscipedia (DmePb, fruit fly, CAA45272),<br />
Drosophila virilis, Antennapedia (DviAnt, fruit fly, AAQ67266.1), Mus musculus, HoxA9<br />
(MmuHoxA9, mouse, NP_034586.1), Neanthes virens, Engrailed (NviEng, annelid, DQ366680).<br />
Dots represent amino acid identity with the amino acid sequence of the T. transversa Cdx<br />
protein shown at the top of the alignment. (B) Alignment tree based on the 49 amino acid<br />
sequences is shaded in Fig. 2A. Algorithm = UPGMA; Bootstrap = 10.000 replicates. Bootstrap<br />
values are given for each node. Due to the small number of residues used in the analysis, the<br />
phylogenetic signal of the tree is limited. The tree shows, however, that TtrCdx clusters with all<br />
other Cdx/caudal protein sequences and thus is a true Cdx protein.<br />
RESULTS<br />
Characterization of the Terebratalia<br />
Not and Cdx genes<br />
The PCR-amplified region of the TtrNot<br />
homeobox encodes for a 45 amino<br />
acids long peptide which is largely<br />
similar to the NveNot sequence of the<br />
cnidarian Nematostella vectensis (82%<br />
sequence identity). This peptide has<br />
clear affinities to the HvuNot protein<br />
of the cnidarian Hydra vulgaris (69%<br />
sequence identity, Fig. 1). The TtrNot<br />
segment cloned by RACE-PCR was<br />
667 base pairs long and is deposited<br />
in GenBank under the accession<br />
number XXXXXX. The transcribed<br />
region downstream of the homeobox<br />
ends with a poly-A stretch and shows<br />
no similarity to other known gene<br />
sequences.<br />
The conserved protein sequence of the<br />
TtrCdx homeobox identified in this study
Chapter IV<br />
Submitted manuscript<br />
69<br />
Fig. 3 Not expression in Terebratalia transversa. Scale bars equal 50 µm, age of specimens<br />
is given in hours after fertilization (hpf) or hours after settlement (hps), respectively. Blue<br />
represents areas of TtrNot expression. (A) Fertilized egg lacking TtrNot expression. (B) 16<br />
cell stage. (C) 32-64 cell stage. (D) Blastula at the onset of gastrulation. TtrNot is expressed<br />
in two fields of cells (arrows) lateral to the future blastopore. (E) Early gastrula in vegetal view<br />
showing the blastopore (asterisk) and two fields of TtrNot expressing cells. (F) Same stage<br />
as in E, lateral view, the blastopore is on the lower side (asterisk). The lateral fields of TtrNot<br />
extend into the animal pole of the gastrula. The ectoderm (ec) and endoderm (en) have started<br />
to form and are demarcated by a dashed line for clarity. (G) The TtrNot expressing cells extend<br />
in a horseshoe-like pattern over the entire gastrula. (H) Lateral view of a late gastrula with<br />
widened archenteron. The TtrNot expressing cells extend over the entire ectoderm (ec) lateral<br />
to the blastopore (asterisk). The lateral fields of TtrNot expressing cells are interconnected via<br />
a small band of TtrNot expressing cells (arrowhead) on the animal pole of the gastrula. TtrNot is<br />
not expressed in the endoderm (en). (I) Slightly elongated gastrula stage, vegetal view showing<br />
the blastopore (asterisk). The position of TtrNot expressing cells has slightly shifted towards<br />
the animal pole, i.e., the future anterior region of the larva. (J) Further elongated gastrula with<br />
blastopore (asterisk) and two fields of TtrNot expressing cells, which are interconnected by a<br />
narrow band of TtrNot expressing cells (arrowhead) in the animal region of the gastrula. (K)
70 Submitted manuscript<br />
Chapter IV<br />
Same stage as in J with animal view onto the future dorso-anterior part of the larva. The two<br />
fields of TtrNot expressing cells which are interconnected by a narrow band of TtrNot expressing<br />
cells (arrowhead) are visible. (L) Elongated gastrula with small blastopore (asterisk). The TtrNot<br />
expressing cells are distributed equally along the posterior part of the future larval apical lobe.<br />
(M) Ventral view of an early three-lobed larva with apical tuft (at), anterior lobe (al), mantle lobe<br />
(ml), and pedicle lobe (pl). The blastopore (asterisk) is closed. The border between ectoderm<br />
(ec) and mesoderm (ms) is visible in the mantle and pedicle lobe. TtrNot expressing cells are<br />
distributed in the posterior part of the apical lobe (indicated by the dashed line). (N) Ventroanterior<br />
view of a larva with all larval lobes fully established: apical lobe (al), mantle lobe (ml),<br />
pedicle lobe (pl). The blastopore (asterisk) is closed and TtrNot expressing cells are distributed<br />
in a ring along the posterior part of the apical lobe. (O) Dorsal view of a fully differentiated<br />
larva with apical lobe (al), mantle lobe (ml), pedicle lobe (pl), and setae (s). TtrNot is no longer<br />
expressed. (P) Posterior view of a juvenile at 360 hours after settlement (hps) with pedicle (p),<br />
anlage of both valves (v) ,and larval setae (s), which extend beyond the valves. TtrNot is not<br />
expressed.<br />
Fig. 4 Cdx expression in<br />
Terebratalia transversa. Scale bars<br />
equal 50 µm, age is given in hours<br />
after fertilization (hpf). (A) Gastrula<br />
stage, TtrCdx (purple) is expressed in<br />
the ectoderm of the posterior pole of<br />
the blastopore (asterisk). (B) Gastrula<br />
stage slightly older than the one in A,<br />
TtrCdx expression on the posterior<br />
side of the blastopore (asterisk) is<br />
more intense than in A. (C) Maximum<br />
projection image of a confocal<br />
reflection scan of a specimen with<br />
similar expression pattern as in B,<br />
showing that TtrCdx is still limited to<br />
the ectoderm. (D) Early larva with<br />
modest expression of TtrCdx (arrow)<br />
in the ventral part of the future mantle<br />
lobe (ml), immediately posterior to<br />
the blastopore (asterisk). The future<br />
apical lobe (al) and posterior lobe (pl)<br />
can already be distinguished. (E) Early<br />
three-lobed larva with apical lobe (al),<br />
mantle lobe (ml), and pedicle lobe<br />
(pl). TtrCdx (arrow) is expressed in<br />
the posterior part of the mantle lobe,<br />
further posterior from the almost closed blastopore (asterisk) than in previous stages. The<br />
larval apical lobe (al), mantle lobe (ml), and pedicle lobe (pl) are further developed. (F) Fully<br />
established larva with expression of TtrCdx (arrow) in the center of the ventral part of the mantle<br />
lobe (ml). Apical lobe (al), mantle lobe, and pedicle lobe (pl) are fully developed and four sets<br />
of larval setae (se) extend from the mantle lobe.
Chapter IV<br />
Submitted manuscript<br />
71<br />
shows 73% -80% sequence similarity<br />
with Cdx/caudal protein sequences<br />
known from other metazoans and 61%<br />
sequence similarity with the Cdx/Xlox<br />
sequence of Nematostella vectensis<br />
(Fig. 2). The TtrCdx segment cloned<br />
by RACE PCR was 1037 base pairs<br />
long and is deposited in GeneBank<br />
under the accession number XXXX.<br />
The transcribed region downstream of<br />
the homeobox ends with a poly-A tail<br />
and shows no similarity to other known<br />
gene sequences.<br />
Not and Cdx expression during<br />
Terebratalia development<br />
Cleavage in Terebratalia transversa<br />
is radial. Within eight hours after<br />
fertilization (hpf), the embryo develops<br />
into a blastula. Gastrulation starts at 18<br />
hpf. The spherical gastrula has a central<br />
blastopore until approximately 26 hpf.<br />
Thereafter, the gastrula elongates and<br />
the blastopore narrows to a slit, moves<br />
anteriorly, and closes at around 42 hpf.<br />
At this stage, the elongated larva starts<br />
to develop its characteristic three–<br />
lobed body, consisting of an anterior<br />
lobe, a mantle lobe, and a pedicle<br />
lobe. At around 65 hpf, the larval setae<br />
start to form and larval development is<br />
complete by approximately 80-96 hpf.<br />
Under our experimental conditions,<br />
larvae settled between 120 and 240<br />
hpf. The expression patterns of TtrNot<br />
and TtrCdx are shown in Figs. 3–5.<br />
Fertilized eggs and early cleavage<br />
stages did not reveal the presence of<br />
a TtrNot RNA transcript (Fig. 3A-C).<br />
Expression of TtrNot starts laterally<br />
on both sides of the blastopore in<br />
the early gastrula stage at 18 hpf<br />
(Fig. 3D). At 22 hpf, the expression<br />
of TtrNot increases in the ectoderm<br />
on both sides of the blastopore and<br />
extends towards the animal pole of the<br />
gastrula (Figs. 3E, F, 5A). These lateral<br />
bands of TtrNot expressing cells fuse<br />
in slightly older stages (i.e., at 24 hpf;<br />
Fig. 3G). Shortly after that, at 26 hpf,<br />
when the archenteron is enlarged, the<br />
band of TtrNot expressing cells on the<br />
animal pole narrows (Figs. 3H, 5B). At<br />
28 hpf the blastopore starts to become<br />
slit-like, the gastrula elongates, and<br />
TtrNot expressing cells are present in<br />
two fields lateral of the blastopore and<br />
close to the future anterior pole of the<br />
larva. The fields of TtrNot expressing<br />
cells are interconnected by a narrow<br />
band of TtrNot expressing cells on the<br />
animal side (Figs. 3I-K, 5C). At around<br />
36 hpf the blastopore starts to close,<br />
the larva elongates further, and TtrNot<br />
expressing cells are only detected<br />
in the animal region of the gastrula,<br />
which later forms the larval apical lobe<br />
(Fig. 3L). Subsequently, the blastopore<br />
closes completely and the larva<br />
differentiates the three characteristic<br />
body lobes. TtrNot is continuously<br />
expressed in a ring of cells in the<br />
apical lobe until the beginning of setae<br />
formation. The area of the apical lobe<br />
where TtrNot is expressed corresponds<br />
to the region that bears the cilia used<br />
for swimming of the larva (Figs. 3M,<br />
N, 5D). Once the larval lobes are fully<br />
established and setae formation has<br />
started (i.e., at around 75 hpf), TtrNot<br />
is no longer detectable (Figs. 3O, 5E).<br />
Likewise, specimens that have settled<br />
and started to metamorphose do not<br />
show any TtrNot expression (Figs. 3P,<br />
5F).<br />
TtrCdx starts to be expressed in<br />
the ectoderm of the gastrula at the<br />
posterior pole of the blastopore (Figs.
72 Submitted manuscript<br />
Chapter IV<br />
Fig. 5 Schematic representation of Not and Cdx expression in Terebratalia transversa.<br />
Dark grey – ectoderm, light grey – endoderm. All specimens are approximately 120 µm in<br />
diameter. (A) Almost spherical gastrula at 22 hours after fertilization (hpf). TtrNot is expressed<br />
in two lateral fields of the ectoderm. TtrCdx is expressed on the posterior side of the blastopore.<br />
(B) Gastrula at 26 hpf. The two lateral fields of TtrNot expressing cells are interconnected by<br />
a narrow band of TtrNot expressing cells on the animal side of the gastrula. TtrCdx is more<br />
intensely expressed posterior to the blastopore. (C) Early larva at 30 hpf. The fields of TtrNot<br />
expressing cells are positioned further towards the animal pole of the gastrula than in previous<br />
stages. The blastopore is still open. TtrCdx is expressed posterior to the blastopore. (D) Larva<br />
at the onset of lobe formation at 42 hpf. TtrNot is solely expressed in the posterior part of the<br />
apical lobe. Locomotory cilia and an apical tuft are already present. TtrCdx is expressed in the<br />
postero-ventral part of the mantle lobe. (E) Three-lobed larva at 75 hpf. TtrNot is no longer<br />
expressed but TtrCdx is still present in the posterior part of the mantle lobe. (F) Juvenile at<br />
360 hours after settlement. The lophophore has started to develop between the valves. Neither<br />
TtrNot nor TtrCdx are expressed.<br />
4A, 5A). TtrCdx expression remains<br />
in this position in the larval ectoderm<br />
throughout development. Expression<br />
intensifies as the gastrula gets older<br />
(Figs. 4B, C, 5B). In early larvae prior<br />
to the onset of lobe formation (Figs.<br />
4D, 5C), early three-lobed larvae (Figs.<br />
4E, 5D), and fully developed larvae<br />
(Figs. 4F, 5E) TtrCdx is expressed in<br />
the ventral ectoderm posterior to the<br />
closed blastopore.<br />
Neither the expression of TtrNot nor<br />
the expression of TtrCdx is co-located<br />
with the larval serotonergic nervous<br />
system of Terebratalia transversa,<br />
which comprises an apical organ with<br />
eight flask-shaped serotonergic cells<br />
that lie antero-dorsally in the apical<br />
lobe (Fig. 5). The flask-shaped cells<br />
are connected via individual neurites<br />
to a larval neuropil that is situated in<br />
the center of the apical lobe. (Fig. 5A,
Chapter IV<br />
Submitted manuscript<br />
73<br />
Fig. 6 Larval<br />
serotonergic nervous<br />
system of Terebratalia<br />
transversa.<br />
Maximum projections of<br />
a confocal microscopy<br />
image stack. Serotonin<br />
is labeled red, tubulin<br />
is green, cell nuclei are<br />
blue. (A) Lateral view<br />
of a fully established<br />
larva with apical lobe<br />
(al), mantle lobe (ml), and pedicle lobe (pl). The apical organ comprises eight flask-shaped<br />
cells (arrows) which are connected by neurites (empty arrowheads) to a larval anterior neuropil<br />
(asterisk). The apical organ is situated towards the dorsal side of the apical lobe. Note that<br />
only cilia but no neural structures are labeled by the α-tubulin antibody. (B) Detailed view of<br />
the apical organ of the specimen shown in A. The apical organ comprises two sets of four<br />
flask-shaped cells each (arrowheads). The flask-shaped cells are connected to the neuropil<br />
(asterisk) by individual neurites (empty arrowheads).<br />
B). Staining with the pan-neural marker<br />
anti-acetylated tubulin did not reveal<br />
any additional neural structures.<br />
DISCUSSION<br />
The role of the Not gene in metazoan<br />
neurogenesis<br />
The designation of the gene “Not”<br />
refers to the site where its expression<br />
was detected for the first time, namely<br />
in the notochord of the African Clawed<br />
Frog, Xenopus laevis (Gont et al.<br />
1993, von Dassow et al. 1993). The<br />
notochord is a cartilaginous, rod<br />
shaped structure, apomorphic to the<br />
Chordata. It is at least present in some<br />
developmental stages of all chordates,<br />
including the ascidian tadpole larva,<br />
and functions as axial skeleton,<br />
induces the development of the<br />
neural tube, and is thus a key player<br />
in chordate neurogenesis (Stemple<br />
2005). Moreover, Not is expressed in<br />
the neural tube of the mouse, chick,<br />
frog, and zebrafish (Stein and Kessel<br />
1995, Talbot et al. 1995, Yasuo and<br />
Lemaire 2001, Beckers et al. 2007).<br />
Where it has been analyzed in detail,<br />
Not seems to act primarily as a<br />
transcriptional repressor (Yasuo and<br />
Lemaire 2001). In zebrafish, loss-offunction<br />
mutants of floating head (flh;<br />
the zebrafish Not homolog) lack the<br />
notochord altogether, and the somites<br />
fuse below the neural tube (Talbot et al.<br />
1995). Expression studies suggest that<br />
cells lacking flh expression differentiate<br />
into muscle rather than notochordal<br />
tissue (Halpern et al. 1995). Noto,<br />
the Not homolog in the mouse, and<br />
flh repress paraxial mesoderm fate<br />
while maintaining axial mesoderm fate<br />
(Amacher and Kimmel 1998).<br />
Only little is known about the<br />
expression patterns and functions of<br />
Not in non-chordate metazoans. In<br />
Trichoplax adhaerens, the Not gene is<br />
expressed at the bottom of body folds<br />
of intact animals as well as during<br />
wound healing (Martinelli and Spring
74 Submitted manuscript<br />
Chapter IV<br />
2004). In addition, Not expressing cells<br />
in this species, which lacks a nervous<br />
system and even neurons, overlaps<br />
with the site of expression of the<br />
neurotransmitter RFamide (Schuchert<br />
1993). In Drosophila, the Not homolog<br />
90Bre is present in the nervous<br />
system, where it is expressed in the<br />
ventral nerve cord and the posterior<br />
brain anlage of germ band retracted<br />
embryos, as well as in the lateral/<br />
posterior region of the eye/antennal<br />
disc (Dessain and McGinnis 1993).<br />
In the ascidians Halocynthia roretzi<br />
and Ciona intestinalis, Hr-Not and Ci-<br />
Not, respectively, are expressed in the<br />
posterior end of the tail, as well as in<br />
the notochord and a small part of the<br />
anterior neural tube in the larval tailbud<br />
stage (Utsumi et al. 2004). These data<br />
hint towards an ancestral role of Not in<br />
metazoan neurogenesis.<br />
The ring-like expression of TtrNot in<br />
the ciliated region of the apical lobe<br />
in Terebratalia larvae is intriguing.<br />
Noto, the Not ortholog in the mouse,<br />
is known to function in ciliogenesis,<br />
and TtrNot might thus serve a similar<br />
role in our study species. In this<br />
context,it should also be considered<br />
that the putative spiralian homolog<br />
of this larval swimming device, the<br />
prototroch, is underlain, and probably<br />
innervated, by a ring nerve (Wanninger<br />
2009). Accordingly, it is tempting<br />
to speculate that Not may also be<br />
expressed in the prototroch ring nerve<br />
of spiralian trochophore larvae and/<br />
or the prototroch itself. In Terebratalia<br />
transversa, however, we did not find<br />
any corresponding neural structure in<br />
the region of Not expression (Fig. 6).<br />
The larval nervous system of T.<br />
transversa differs in several details<br />
from that of the craniiform brachiopod<br />
Novocrania anomola. In N. anomala,<br />
the apical organ comprises four,<br />
centrally positioned serotonergic flaskshaped<br />
cells that are connected to two<br />
ventral neurites which elongate laterally<br />
along the larval body (Altenburger<br />
and Wanninger 2010). By contrast,<br />
the apical organ of T. transversa has<br />
two sets of flask-shaped cells. Each<br />
set contains four cells and each cell is<br />
connected to the larval anterior neuropil<br />
by a single serotonergic neurite. Due to<br />
these differences, the morphology of<br />
the ancestral brachiopod larval apical<br />
organ remains elusive. However, the<br />
data currently available indicate that an<br />
apical organ comprising serotonergic<br />
flask-shaped cells was part of the<br />
brachiopod ground pattern and most<br />
likely constitutes a morphological<br />
apomorphy of Lophotrochzoa, since<br />
such cells are also found in larval<br />
Entoprocta, Mollusca, Nemertea,<br />
Annelida, and Ectoprocta (Pires and<br />
Woollacott 1997, Shimizu et al. 2000,<br />
Friedrich et al. 2002, Voronezhskaya<br />
et al. 2002, 2003, McDougall et al.<br />
2006, Wanninger et al. 2007, Fuchs<br />
and Wanninger 2008, Chernyshev<br />
and Magarlamov 2010, Nielsen and<br />
Worsaae 2010).<br />
Not expression during gastrulation<br />
and germ layer formation<br />
In all species studied so far, Not<br />
expression starts prior to or at the onset<br />
of gastrulation. This is also the case<br />
in the brachiopod investigated herein,<br />
Terebratalia transversa. In the sea<br />
urchin Strongylocentrotus purpuratus,<br />
Not is expressed in the vegetal plate at<br />
the mesenchyme-blastula stage and<br />
in the secondary mesenchyme, with
Chapter IV<br />
Submitted manuscript<br />
75<br />
expression ceasing after gastrulation<br />
(Peterson et al. 1999). In ascidians,<br />
Not expression starts at the eight<br />
cell stage in all blastomeres and is<br />
thereafter expressed in the posterior<br />
part of the larval tail, the notochord,<br />
and a small part of the anterior neural<br />
tube at the tailbud stage (Utsumi<br />
et al. 2004). Interestingly, we found<br />
TtrNot being solely expressed in the<br />
ectoderm of T. transversa, while their<br />
homologs are expressed in all three<br />
germ layers during Xenopus and<br />
ascidian embryogenesis (von Dassow<br />
et al. 1993, Utsumi et al. 2004).<br />
Apart from the development of the<br />
nervous system, the notochord,<br />
and various germ layers, Not is also<br />
responsible for left/right patterning<br />
in the mouse, where it is expressed<br />
in the “node”, i.e., the organizer of<br />
gastrulation (Beckers et al. 2007).<br />
In the sea urchins Hemicentrotus<br />
pulcherimus and Strongylocentrotus<br />
purpuratus, Not is expressed in the<br />
archenteron of the gastrula and in<br />
the mesoderm of the right coelomic<br />
pouch of two-armed pluteus larvae,<br />
were it is likewise involved in left/right<br />
determination (Peterson et al. 1999,<br />
Hibino et al. 2006).<br />
The current data suggest an overall role<br />
of Not in gastrulation as well as germ<br />
layer and nervous system patterning.<br />
Whether Not was used in specification<br />
of all three germ layers in Urbilateria<br />
(as exemplified in the ascidians and<br />
Xenopus) or whether its ancestral role<br />
was in ectoderm patterning alone (as<br />
in Terebratalia) remains to be revealed<br />
by future comparative studies. In<br />
any case, it appears that the Not<br />
gene has been co-opted into several<br />
other functions during evolution of<br />
respective metazoan (deuterostome)<br />
lineages, such as notochord formation<br />
in chordates and left/right patterning<br />
in ambulacrarians (sea urchin) and<br />
vertebrates (mouse).<br />
The role of Cdx in metazoan<br />
development<br />
Cdx is a member of the ParaHox cluster<br />
in which three genes are linked in a<br />
manner reminiscent of the Hox genes,<br />
with the gene order 3’-Gsx-Xlox-Cdx-5’<br />
(Brooke et al. 1998). Compared to Hox<br />
genes, ParaHox genes seem to be<br />
much more evolutionary labile, since<br />
they do not appear together in all<br />
species investigated and sometimes<br />
they are not clustered (Ferrier and<br />
Holland 2002).<br />
Cdx expression patterns are known<br />
from several animal phyla and there is<br />
a wide range of tissues in which Cdx is<br />
expressed (Fröbius and Seaver 2006).<br />
A gene related to Cdx is present in<br />
the proposed bilaterian sister group,<br />
the cnidarian Nematostella vectensis<br />
(Chourrout et al. 2006, Ryan et al. 2006,<br />
2007). Cdx was first characterized as a<br />
posterior patterning gene in Drosophila<br />
melanogaster (Mlodzik et al. 1985)<br />
and it appears to serve a similar role<br />
in a number of other taxa including<br />
various arthropods, the nematode<br />
Caenorhabditis elegans, and the basal<br />
gastropod mollusk Patella vulgata<br />
(Waring and Kenyon 1991, Xu et al.<br />
1994, Schulz et al. 1998, Abzhanov<br />
and Kaufman 2000, Dearden and<br />
Akam 2001, Rabet et al. 2001, Copf et<br />
al. 2003, Le Gouar et al. 2003, 2004,<br />
Shinmyo et al. 2005, Olesnicky et al.<br />
2006). In the annelids Platynereis<br />
dumerilii, Nereis virens, Tubifex<br />
tubifex, and Capitella sp., Cdx has an
76 Submitted manuscript<br />
Chapter IV<br />
anterior and a posterior expression<br />
domain (Fröbius and Seaver 2006,<br />
Matsuo and Shimizu 2006, Kulakova<br />
et al. 2008, Hui et al. 2009).<br />
The expression pattern of Cdx in<br />
Terebratalia transversa shows some<br />
similarity to that of Platynereis dumerilii.<br />
In both species Cdx is expressed in the<br />
ectoderm at the onset of gastrulation. In<br />
P. dumerilii, the ectodermal expression<br />
of PduCdx encircles the posterior<br />
portion of the slit-like blastopore and<br />
extends from there anteriorly along its<br />
edges (de Rosa et al. 2005). PduCdx<br />
continues to be expressed in the<br />
posterior part of the trochophore larva<br />
in the posterior midgut and hindgut<br />
(Hui et al. 2009). Expression of Cdx in<br />
the gut is also found in the sea urchin<br />
Strongylocentrotus purpuratus, the<br />
lancelet Brachiostoma floridae, and<br />
the mouse Mus musculus (Duprey<br />
et al. 1988, Brooke et al. 1998,<br />
Arnone et al. 2006). We did not find<br />
expression of TtrCdx in the larval gut<br />
of Terebratalia transversa, which might<br />
be due to the fact that those larvae are<br />
lecithotrophic and that metamorphosis<br />
is catastrophic, i.e., that all major larval<br />
tissues degenerate after settlement<br />
(Stricker and Reed 1985a, 1985b).<br />
Since we did not investigate feeding<br />
juveniles with a functional gut, the role<br />
of TtrCdx in gut formation remains<br />
elusive.<br />
Concerning the role of Cdx in the<br />
protostome-deuterostome ancestor<br />
(PDA), two major hypotheses are<br />
currently discussed. Either, expression<br />
in the PDA might have been in an<br />
anterior and a posterior domain of the<br />
nervous system, as in recent acoels<br />
as well as the lophotrochozoans<br />
Platynereis dumerilii, Capitella sp.,<br />
Tubifex tubifex, and Patella vulgata<br />
(Le Gouar et al. 2003, de Rosa et al.<br />
2005, Matsuo et al. 2005, Fröbius and<br />
Seaver 2006, Hejnol and Martindale<br />
2008). Expression in the hindgut and<br />
posterior tissues of recent animals<br />
would thus have been co-opted. Or,<br />
Cdx expression in the PDA was in<br />
posterior tissues and a dissociation<br />
of Cdx from the ParaHox cluster in<br />
Lophotrochozoa allowed for its cooption<br />
into the anterior domain of the<br />
nervous system, as is the case in<br />
acoels and some lophotrochozoans<br />
(Hui et al. 2009). In this respect it<br />
would be interesting to focus future<br />
investigations on the genomic<br />
arrangement and the expression of<br />
the respective Gsx and Xlox genes of<br />
the ParaHox cluster in brachiopods.<br />
ACKNOWLEDGEMENTS<br />
We thank the Friday Harbor<br />
Laboratories and especially Billie<br />
Swalla (University of Washington) for<br />
providing lab space and assistance in<br />
rearing Terebratalia transversa. Olga<br />
Lévai (Leica Microsystems, Mannheim,<br />
Germany) is thanked for providing the<br />
SP5 confocal system that was used for<br />
the scans upon which Fig. 6 is based.<br />
We are grateful to Marta Chiodin<br />
(University of Barcelona) for guidance<br />
in lab procedures. Bernard M. Degnan<br />
(University of Queensland) is thanked<br />
for sharing previously unpublished<br />
sequence data of the demosponge<br />
Amphimedon queenslandica. This<br />
study was funded by the Danish<br />
Agency for Science, Technology and<br />
Innovation (grant no. 645-06-0294<br />
to AW). Research in the lab of AW<br />
and PM was further supported by<br />
the EU-funded Marie Curie Network
Chapter IV<br />
Submitted manuscript<br />
77<br />
MOLMORPH (contract grant number<br />
MEST-CT-2005-020542). PM is<br />
grateful to the Spanish Ministerio<br />
de Ciencia e Innovación and the<br />
Generalitat de Catalunya for financial<br />
support.<br />
REFERENCES<br />
Abdelkhalek, H. B., A. Beckers,<br />
K. Schuster-Gossler, M.<br />
N. Pavlova, H. Burkhardt,<br />
H. Lickert, J. Rossant, R.<br />
Reinhardt, L. C. Schalkwyk,<br />
I. Müller, B. G. Herrmann,<br />
M. Ceolin, R. Rivera-Pomar,<br />
and A. Gossler. 2004. The<br />
mouse homeobox gene Not is<br />
required for caudal notochord<br />
development and affected by<br />
the truncate mutation. Genes<br />
Dev 18:1725-1736.<br />
Abzhanov, A., and T. C. Kaufman.<br />
2000. Embryonic expression<br />
patterns of the Hox genes of the<br />
crayfish Procambarus clarkii<br />
(Crustacea, Decapoda). Evol<br />
Dev 2:271-283.<br />
Altenburger, A., and A. Wanninger.<br />
2010. Neuromuscular<br />
development in Novocrania<br />
anomala: evidence for the<br />
presence of serotonin and<br />
a spiralian-like apical organ<br />
in lecithotrophic brachiopod<br />
larvae. Evol Dev 12:16-24.<br />
Amacher, S. L., and C. B. Kimmel.<br />
1998. Promoting notochord<br />
fate and repressing muscle<br />
development in zebrafish axial<br />
mesoderm. Development<br />
125:1397-1406.<br />
Arnone, M. I., F. Rizzo, R. Annunciata,<br />
R. A. Cameron, K. J. Peterson,<br />
and P. Martínez. 2006. Genetic<br />
organization and embryonic<br />
expression of the ParaHox<br />
genes in the sea urchin S.<br />
purpuratus: Insights into the<br />
relationship between clustering<br />
and colinearity. Dev Biol 300:63-<br />
73.<br />
Beckers, A., L. Alten, C. Viebahn,<br />
P. Andre, and A. Gossler.<br />
2007. The mouse homeobox<br />
gene Noto regulates node<br />
morphogenesis, notochordal<br />
ciliogenesis, and left-right<br />
patterning. Proc Natl Acad Sci<br />
USA 104:15765-15770.<br />
Brooke, N. M., J. Garcia-Fernandez,<br />
and P. W. H. Holland. 1998.<br />
The ParaHox gene cluster is an<br />
evolutionary sister of the Hox<br />
gene cluster. Nature 392:920-<br />
922.<br />
Chernyshev, A. V., and T. Y.<br />
Magarlamov. 2010. The first<br />
data on the nervous system<br />
of hoplonemertean larvae<br />
(Nemertea, Hoplonemertea).<br />
Gen Biol 430:48-50.<br />
Chourrout, D., F. Delsuc, P. Chourrout,<br />
R. B. Edvardsen, F. Rentzsch,<br />
E. Renfer, M. F. Jensen, B.<br />
Zhu, P. de Jong, R. E. Steele,<br />
and U. Technau. 2006. Minimal<br />
ProtoHox cluster inferred from<br />
bilaterian and cnidarian Hox<br />
complements. Nature 442:684-<br />
687.<br />
Cohen, B. L., and A. Weydmann.<br />
2005. Molecular evidence that<br />
phoronids are a subtaxon of<br />
brachiopods (Brachiopoda:<br />
Phoronata) and that genetic<br />
divergence of metazoan phyla<br />
began long before the early
78 Submitted manuscript<br />
Chapter IV<br />
Cambrian. Org Divers Evol<br />
5:253-273.<br />
Copf, T., N. Rabet, S. E. Celniker,<br />
and M. Averof. 2003. Posterior<br />
patterning genes and the<br />
identification of a unique<br />
body region in the brine<br />
shrimp Artemia franciscana.<br />
Development 130:5915-5927.<br />
Copf, T., R. Schröder, and M. Averof.<br />
2004. Ancestral role of caudal<br />
genes in axis elongation and<br />
segmentation. Proc Natl Acad<br />
Sci USA 101:17711-17715.<br />
de Rosa, R., J. K. Grenier, T. Andreeva,<br />
C. E. Cook, A. Adoutte, M.<br />
Akam, S. B. Carroll, and G.<br />
Balavoine. 1999. Hox genes in<br />
brachiopods and priapulids and<br />
protostome evolution. Nature<br />
399:772-776.<br />
de Rosa, R., B. Prud’homme, and G.<br />
Balavoine. 2005. caudal and<br />
even-skipped in the annelid<br />
Platynereis dumerilii and the<br />
ancestry of posterior growth.<br />
Evol Dev 7:574-587.<br />
Dearden, P. K., and M. Akam. 2001.<br />
Early embryo patterning in the<br />
grasshopper, Schistocerca<br />
gregaria:<br />
wingless,<br />
decapentaplegic and caudal<br />
expression. Development<br />
128:3435-3444.<br />
Dessain, S., and W. McGinnis. 1993.<br />
Drosophila homeobox genes.<br />
Adv Dev Biochem 2:1-55.<br />
Duprey, P., K. Chowdhury, G. R.<br />
Dressler, R. Balling, D. Simon, J.<br />
L. Guenet, and P. Gruss. 1988.<br />
A mouse gene homologous to<br />
the Drosophila gene caudal is<br />
expressed in epithelial cells<br />
from the embryonic intestine.<br />
Genes Dev 2:1647-1654.<br />
Epstein, M., G. Pillemer, R. Yelin, J. K.<br />
Yisraeli, and A. Fainsod. 1997.<br />
Patterning of the embryo along<br />
the anterior-posterior axis:<br />
the role of the caudal genes.<br />
Development 124:3805-3814.<br />
Ferrier, D. E. K., and P. W. H. Holland.<br />
2002. Ciona intestinalis<br />
ParaHox genes: evolution of<br />
Hox/ParaHox cluster integrity,<br />
developmental mode, and<br />
temporal colinearity. Mol<br />
Phylogenet Evol 24:412-417.<br />
Friedrich, S., A. Wanninger, M.<br />
Bruckner, and G. Haszprunar.<br />
2002. Neurogenesis in the<br />
mossy chiton, Mopalia muscosa<br />
(Gould) (Polyplacophora):<br />
Evidence against molluscan<br />
metamerism. J Morphol<br />
253:109-117.<br />
Fröbius, A., and E. Seaver. 2006.<br />
ParaHox gene expression in the<br />
polychaete annelid Capitella sp.<br />
I. Dev Genes Evol 216:81-88.<br />
Fuchs, J., and A. Wanninger.<br />
2008. Reconstruction of<br />
the neuromuscular system<br />
of the swimming-type larva<br />
of Loxosomella atkinsae<br />
(Entoprocta) as inferred by<br />
fluorescence labelling and<br />
confocal microscopy. Org<br />
Divers Evol 8:325-335.<br />
Gauchat, D., F. Mazet, C. Berney,<br />
M. Schummer, S. Kreger, J.<br />
Pawlowski, and B. Galliot. 2000.<br />
Evolution of Antp-class genes<br />
and differential expression of<br />
Hydra Hox/ParaHox genes in<br />
anterior patterning. Proc Natl<br />
Acad Sci USA 97:4493-4498.<br />
Gont, L., H. Steinbeisser, B. Blumberg,
Chapter IV<br />
Submitted manuscript<br />
79<br />
and E. de Robertis. 1993. Tail<br />
formation as a continuation of<br />
gastrulation: the multiple cell<br />
populations of the Xenopus<br />
tailbud derive from the late<br />
blastopore lip. Development<br />
119:991-1004.<br />
Gont, L. K., A. Fainsod, S.-H. Kim,<br />
and E. M. de Robertis.<br />
1996. Overexpression of the<br />
homeobox gene Xnot-2 leads<br />
to notochord formation in<br />
Xenopus. Dev Biol 174:174-<br />
178.<br />
Halpern, M. E., C. Thisse, R. K. Ho,<br />
B. Thisse, B. Riggleman, B.<br />
Trevarrow, E. S. Weinberg,<br />
J. H. Postlethwait, and C. B.<br />
Kimmel. 1995. Cell-autonomous<br />
shift from axial to paraxial<br />
mesodermal development in<br />
zebrafish floating head mutants.<br />
Development 121:4257-4264.<br />
Hejnol, A., and M. Q. Martindale. 2008.<br />
Acoel development indicates<br />
the independent evolution of<br />
the bilaterian mouth and anus.<br />
Nature 456:382-386.<br />
Hejnol, A., M. Obst, A. Stamatakis,<br />
M. Ott, G. W. Rouse, G. D.<br />
Edgecombe, P. Martinez, J.<br />
Baguñà, X. Bailly, U. Jondelius,<br />
M. Wiens, W. E. G. Müller, E.<br />
Seaver, W. C. Wheeler, M. Q.<br />
Martindale, G. Giribet, and<br />
C. W. Dunn. 2009. Assessing<br />
the root of bilaterian animals<br />
with scalable phylogenomic<br />
methods. Proc R Soc B<br />
276:4261-4270.<br />
Hibino, T., Y. Ishii, M. Levin, and<br />
A. Nishino. 2006. Ion flow<br />
regulates left–right asymmetry<br />
in sea urchin development. Dev<br />
Genes Evol 216:265-276.<br />
Hoskins, R. A., J. W. Carlson, C.<br />
Kennedy, D. Acevedo, M.<br />
Evans-Holm, E. Frise, K. H.<br />
Wan, S. Park, M. Mendez-<br />
Lago, F. Rossi, A. Villasante,<br />
P. Dimitri, G. H. Karpen, and S.<br />
E. Celniker. 2007. Sequence<br />
finishing and mapping of<br />
Drosophila melanogaster<br />
heterochromatin. Science<br />
316:1625-1628.<br />
Hueber, S. D., and I. Lohmann. 2008.<br />
Shaping segments: Hox gene<br />
function in the genomic age.<br />
Bioessays 30:965-979.<br />
Hui, J., F. Raible, N. Korchagina, N.<br />
Dray, S. Samain, G. Magdelenat,<br />
C. Jubin, B. Segurens, G.<br />
Balavoine, D. Arendt, and D.<br />
Ferrier. 2009. Features of the<br />
ancestral bilaterian inferred from<br />
Platynereis dumerilii ParaHox<br />
genes. BMC Biol 7:43.<br />
Knezevic, V., M. Ranson, and S.<br />
Mackem. 1995. The organizerassociated<br />
chick homeobox<br />
gene, Gnot1, is expressed<br />
before gastrulation and<br />
regulated synergistically by<br />
activin and retinoic acid. Dev<br />
Biol 171:458-470.<br />
Kulakova, M., C. Cook, and T.<br />
Andreeva. 2008. ParaHox<br />
gene expression in larval and<br />
postlarval development of<br />
the polychaete Nereis virens<br />
(Annelida, Lophotrochozoa).<br />
BMC Dev Biol 8:61.<br />
Lanfear, R., and L. Bromham. 2008.<br />
Statistical tests between<br />
competing hypotheses of Hox<br />
cluster evolution. Syst. Biol.<br />
57:708-718.
80 Submitted manuscript<br />
Chapter IV<br />
Larroux, C., G. N. Luke, P. Koopman,<br />
D. S. Rokhsar, S. M. Shimeld,<br />
and B. M. Degnan. 2008.<br />
Genesis and expansion of<br />
metazoan transcription factor<br />
gene classes. Mol Biol Evol<br />
25:980-996.<br />
Le Gouar, M., N. Lartillot, A. Adoutte,<br />
and M. Vervoort. 2003.<br />
The expression of a caudal<br />
homologue in a mollusc, Patella<br />
vulgata. Gene Expr Patterns.<br />
3:35-37.<br />
Macdonald, P. M., and G. Struhl. 1986.<br />
A molecular gradient in early<br />
Drosophila embryos and its role<br />
in specifying the body pattern.<br />
Nature 324:537-545.<br />
Martinelli, C., and J. Spring. 2004.<br />
Expression pattern of the<br />
homeobox gene Not in the<br />
basal metazoan Trichoplax<br />
adhaerens. Gene Expr Patterns.<br />
4:443-447.<br />
Matsuo, K., and T. Shimizu. 2006.<br />
Embryonic expression of a<br />
decapentaplegic gene in the<br />
oligochaete annelid Tubifex<br />
tubifex. Gene Expr Patterns.<br />
6:800-806.<br />
Matsuo, K., H. Yoshida, and T. Shimizu.<br />
2005. Differential expression<br />
of caudal and dorsal genes in<br />
the teloblast lineages of the<br />
oligochaete annelid Tubifex<br />
tubifex. Dev Genes Evol<br />
215:238-247.<br />
McDougall, C., W.-C. Chen, S.<br />
Shimeld, and D. Ferrier.<br />
2006. The development of<br />
the larval nervous system,<br />
musculature and ciliary bands<br />
of Pomatoceros lamarckii<br />
(Annelida): heterochrony in<br />
polychaetes. Front Zool 3:16.<br />
McGinnis, W., M. S. Levine, E. Hafen,<br />
A. Kuroiwa, and W. J. Gehring.<br />
1984. A conserved DNA<br />
sequence in homoeotic genes<br />
of the Drosophila Antennapedia<br />
and bithorax complexes. Nature<br />
308:428-433.<br />
Mlodzik, M., A. Fjose, and W. J.<br />
Gehring. 1985. Isolation of<br />
caudal, a Drosophila homeo<br />
box-containing gene with<br />
maternal expression, whose<br />
transcripts form a concentration<br />
gradient at the pre-blastoderm<br />
stage. EMBO J 4:2961-2969.<br />
Nielsen, C. 2002. Ciliary filter-feeding<br />
structures in adult and larval<br />
gymnolaemate bryozoans.<br />
Invertebr Biol 121:255-261.<br />
Nielsen, C., and K. Worsaae. 2010.<br />
Structure and occurrence of<br />
cyphonautes larvae (Bryozoa,<br />
Ectoprocta). J Morphol<br />
271:1094-1109.<br />
Odenthal, J., P. Haffter, E. Vogelsang,<br />
M. Brand, F. van Eeden, M.<br />
Furutani-Seiki, M. Granato,<br />
M. Hammerschmidt, C.<br />
Heisenberg, Y. Jiang, D. Kane,<br />
R. Kelsh, M. Mullins, R. Warga,<br />
M. Allende, E. Weinberg,<br />
and C. Nüsslein-Volhard.<br />
1996. Mutations affecting the<br />
formation of the notochord<br />
in the zebrafish, Danio rerio.<br />
Development 123:103-115.<br />
Olesnicky, E. C., A. E. Brent, L. Tonnes,<br />
M. Walker, M. A. Pultz, D.<br />
Leaf, and C. Desplan. 2006. A<br />
caudal mRNA gradient controls<br />
posterior development in the<br />
wasp Nasonia. Development<br />
133:3973-3982.
Chapter IV<br />
Submitted manuscript<br />
81<br />
Paps, J., J. Baguñà, and M. Riutort.<br />
2009. Lophotrochozoa internal<br />
phylogeny: new insights from an<br />
up-to-date analysis of nuclear<br />
ribosomal genes. Proc R Soc B<br />
276:1245-1254.<br />
Peterson, K. J., Y. Harada, R. A.<br />
Cameron, and E. H. Davidson.<br />
1999. Expression pattern of<br />
Brachyury and Not in the sea<br />
urchin: comparative implications<br />
for the origins of mesoderm in<br />
the basal deuterostomes. Dev<br />
Biol 207:419-431.<br />
Pires, A., and R. M. Woollacott. 1997.<br />
Serotonin and dopamine have<br />
opposite effects on phototaxis<br />
in larvae of the bryozoan Bugula<br />
neritina. Biol Bull 192:399-409.<br />
Quiquand, M., N. Yanze, J. Schmich,<br />
V. Schmid, B. Galliot, and S.<br />
Piraino. 2009. More constraint<br />
on ParaHox than Hox gene<br />
families in early metazoan<br />
evolution. Dev Biol 328:173-<br />
187.<br />
Rabet, N., J.-M. Gibert, É. QuÉinnec,<br />
J. S. Deutsch, and E. Mouchel-<br />
Vielh. 2001. The caudal gene of<br />
the barnacle Sacculina carcini<br />
is not expressed in its vestigial<br />
abdomen. Dev Genes Evol<br />
211:172-178.<br />
Ryan, J., P. Burton, M. Mazza, G.<br />
Kwong, J. Mullikin, and J.<br />
Finnerty. 2006. The cnidarianbilaterian<br />
ancestor possessed<br />
at least 56 homeoboxes:<br />
evidence from the starlet<br />
sea anemone, Nematostella<br />
vectensis. Genome Biol 7:R64.<br />
Ryan, J. F., M. E. Mazza, K. Pang,<br />
D. Q. Matus, A. D. Baxevanis,<br />
M. Q. Martindale, and J. R.<br />
Finnerty. 2007. Pre-bilaterian<br />
origins of the Hox cluster and<br />
the Hox code: evidence from<br />
the sea anemone, Nematostella<br />
vectensis. PLoS ONE 2.<br />
Schuchert, P. 1993. Trichoplax<br />
adhaerens (phylum Placozoa)<br />
has cells that react with<br />
antibodies against the<br />
neuropeptide RFamide. Acta<br />
Zool 74.<br />
Schulz, C., R. Schröder, B. Hausdorf,<br />
C. Wolff, and D. Tautz. 1998. A<br />
caudal homologue in the short<br />
germ band beetle Tribolium<br />
shows similarities to both, the<br />
Drosophila and the vertebrate<br />
caudal expression patterns.<br />
Dev Genes Evol 208:283-289.<br />
Scott, M. P., and A. J. Weiner. 1984.<br />
Structural relationships among<br />
genes that control development:<br />
sequence homology between<br />
the antennapedia, ultrabithorax,<br />
and fushi tarazu loci of<br />
Drosophila. Proc Natl Acad Sci<br />
USA 81:4115-4119.<br />
Shimizu, K., E. Hunter, and N.<br />
Fusetani. 2000. Localisation<br />
of biogenic amines in larvae<br />
of Bugula neritina (Bryozoa:<br />
Cheilostomatida) and their<br />
effects on settlement. Mar Biol<br />
136:1-9.<br />
Shinmyo, Y., T. Mito, T. Matsushita,<br />
I. Sarashina, K. Miyawaki, H.<br />
Ohuchi, and S. Noji. 2005.<br />
caudal is required for gnathal<br />
and thoracic patterning and<br />
for posterior elongation in the<br />
intermediate-germband cricket<br />
Gryllus bimaculatus. Mech Dev<br />
122:231-239.<br />
Srivastava, M., O. Simakov, J.
82 Submitted manuscript<br />
Chapter IV<br />
Chapman, B. Fahey, M. E.<br />
A. Gauthier, T. Mitros, G.<br />
S. Richards, C. Conaco, M.<br />
Dacre, U. Hellsten, C. Larroux,<br />
N. H. Putnam, M. Stanke, M.<br />
Adamska, A. Darling, S. M.<br />
Degnan, T. H. Oakley, D. C.<br />
Plachetzki, Y. Zhai, M. Adamski,<br />
A. Calcino, S. F. Cummins, D.<br />
M. Goodstein, C. Harris, D. J.<br />
Jackson, S. P. Leys, S. Shu,<br />
B. J. Woodcroft, M. Vervoort,<br />
K. S. Kosik, G. Manning, B. M.<br />
Degnan, and D. S. Rokhsar.<br />
2010. The Amphimedon<br />
queenslandica genome and the<br />
evolution of animal complexity.<br />
Nature 466:720-726.<br />
Stein, S., and M. Kessel. 1995. A<br />
homeobox gene involved in<br />
node, notochord and neural<br />
plate formation of chick<br />
embryos. Mech Dev 49:37-48.<br />
Stein, S., K. Niß, and M. Kessel. 1996.<br />
Differential activation of the<br />
clustered homeobox genes<br />
CNOT2 and CNOT1 during<br />
notogenesis in the chick. Dev<br />
Biol 180:519-533.<br />
Stemple, D. L. 2005. Structure and<br />
function of the notochord: an<br />
essential organ for chordate<br />
development. Development<br />
132:2503-2512.<br />
Stricker, S. A., and C. G. Reed. 1985a.<br />
The ontogeny of shell secretion<br />
in Terebratalia transversa<br />
(Brachiopoda, Articulata). 1.<br />
Development of the mantle. J<br />
Morphol 183:233-250.<br />
Stricker, S. A., and C. G. Reed. 1985b.<br />
The ontogeny of shell secretion<br />
in Terebratalia transversa<br />
(Brachiopoda, Articulata). 2.<br />
formation of the protegulum<br />
and juvenile shell. J Morphol<br />
183:251-271.<br />
Talbot, W. S., B. Trevarrow, M. E.<br />
Halpern, A. E. Melby, G.<br />
Farr, J. H. Postlethwait, T.<br />
Jowett, C. B. Kimmel, and D.<br />
Kimelman. 1995. A homeobox<br />
gene essential for zebrafish<br />
notochord development. Nature<br />
378:150-157.<br />
Utsumi, N., Y. Shimojima, and H. Saiga.<br />
2004. Analysis of ascidian<br />
Not genes highlights their<br />
evolutionarily conserved and<br />
derived features of structure<br />
and expression in development.<br />
Dev Genes Evol 214:460-465.<br />
von Dassow, G., J. E. Schmidt, and<br />
D. Kimelman. 1993. Induction<br />
of the Xenopus organizer:<br />
expression and regulation of<br />
Xnot, a novel FGF and activinregulated<br />
homeo box gene.<br />
Genes Dev 7:355-366.<br />
Voronezhskaya, E. E., E. B. Tsitrin,<br />
and L. P. Nezlin. 2003.<br />
Neuronal development in<br />
larval polychaete Phyllodoce<br />
maculata (Phyllodocidae). J<br />
Comp Neurol. 455:299-309.<br />
Voronezhskaya, E. E., S. A. Tyurin,<br />
and L. P. Nezlin. 2002. Neuronal<br />
development in larval chiton<br />
Ischnochiton hakodadensis<br />
(Mollusca: Polyplacophora).<br />
J Submicrosc Cytol Pathol<br />
444:25-38.<br />
Wanninger, A. 2009. Shaping the<br />
things to come: ontogeny<br />
of<br />
lophotrochozoan<br />
neuromuscular systems and<br />
the Tetraneuralia concept. Biol.<br />
Bull. 216:293-306.
Chapter IV<br />
Submitted manuscript<br />
83<br />
Wanninger, A., J. Fuchs, and G.<br />
Haszprunar. 2007. Anatomy<br />
of the serotonergic nervous<br />
system of an entoproct creepingtype<br />
larva and its phylogenetic<br />
implications. Invertebr Biol<br />
126:268-278.<br />
Waring, D. A., and C. Kenyon.<br />
1991. Regulation of cellular<br />
responsiveness to inductive<br />
signals in the developing C.<br />
elegans nervous system.<br />
Nature 350:712-715.<br />
Williams, A., and S. J. Carlson. 2007.<br />
Affinities of brachiopods and<br />
trends in their evolution in P.<br />
A. Selden, ed. Treatise on<br />
Invertebrate Paleontology,<br />
Part H, Brachiopoda, Revised.<br />
Geological Society of America<br />
& Paleontological Institute,<br />
Boulder, Colorado & Lawrence,<br />
Kansas.<br />
Xu, X., P. X. Xu, and Y. Suzuki.<br />
1994. A maternal homeobox<br />
gene, Bombyx caudal, forms<br />
both mRNA and protein<br />
concentration gradients<br />
spanning anteroposterior<br />
axis during gastrulation.<br />
Development 120:277-285.<br />
Yasuo, H., and P. Lemaire. 2001.<br />
Role of goosecoid, Xnot<br />
and Wnt antagonists in the<br />
maintenance of the notochord<br />
genetic programme in Xenopus<br />
gastrulae. Development<br />
128:3783-3793.