Chemical Reactions
Chemical Reactions
Chemical Reactions
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
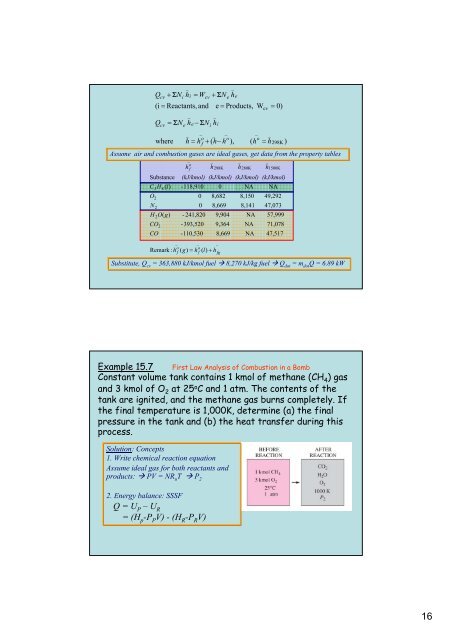
Q<br />
(i = Reactants, and<br />
Q<br />
cv<br />
cv<br />
where<br />
2<br />
_<br />
+ ΣN<br />
h = W<br />
i<br />
_<br />
_<br />
= ΣN<br />
h − ΣN<br />
h<br />
_<br />
o<br />
f<br />
Remark : h<br />
e<br />
i<br />
e<br />
_<br />
o<br />
f<br />
cv<br />
+ ΣN<br />
i<br />
_<br />
e = Products, W<br />
_<br />
_<br />
o<br />
h = h + ( h−<br />
h ),<br />
_<br />
o<br />
f<br />
_<br />
o<br />
f<br />
( g)<br />
= h<br />
_<br />
i<br />
298K<br />
_<br />
( l)<br />
+ h<br />
e<br />
_<br />
h<br />
e<br />
_<br />
o<br />
( h<br />
cv<br />
= 0)<br />
_<br />
= h<br />
Assume air and combustion gases are ideal gases, get data from the property tables<br />
h<br />
h<br />
Substitute, Q cv<br />
= 363,880 kJ/kmol fuel 8,270 kJ/kg fuel Q dot<br />
= m dot<br />
Q = 6.89 kW<br />
_<br />
280K<br />
_<br />
298K<br />
1500K<br />
Substance (kJ/kmol) (kJ/kmol) (kJ/kmol) (kJ/kmol)<br />
C3H8(<br />
l)<br />
-118,910 0 NA NA<br />
O2<br />
0 8,682 8,150 49,292<br />
N2<br />
0 8,669 8,141 47,073<br />
H 2O(<br />
g)<br />
- 241,820 9,904 NA 57,999<br />
CO -393,520 9,364 NA 71,078<br />
CO -110,530 8,669 NA 47,517<br />
fg<br />
h<br />
h<br />
)<br />
Example 15.7 First Law Analysis of Combustion in a Bomb<br />
Constant volume tank contains 1 kmol of methane (CH 4 ) gas<br />
and 3 kmol of O 2 at 25 o C and 1 atm. The contents of the<br />
tank are ignited, and the methane gas burns completely. If<br />
the final temperature is 1,000K, determine (a) the final<br />
pressure in the tank and (b) the heat transfer during this<br />
process.<br />
Solution: Concepts<br />
1. Write chemical reaction equation<br />
Assume ideal gas for both reactants and<br />
products: PV = NR u T P 2<br />
2. Energy balance: SSSF<br />
Q = U P –U R<br />
= (H p -P P V) - (H R -P R V)<br />
16