Chemical Reactions
Chemical Reactions
Chemical Reactions
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
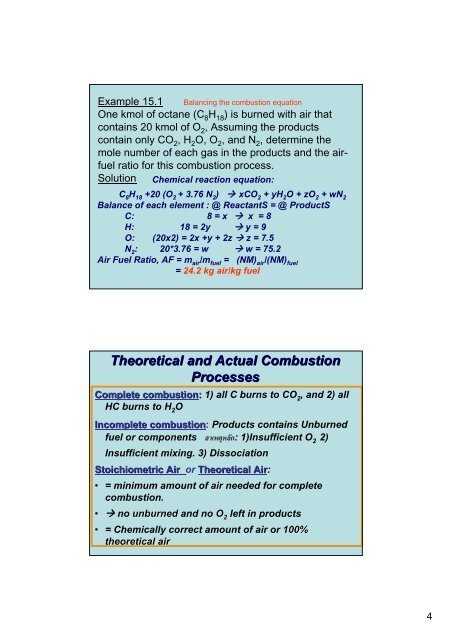
Example 15.1 Balancing the combustion equation<br />
One kmol of octane (C 8 H 18 ) is burned with air that<br />
contains 20 kmol of O 2 , Assuming the products<br />
contain only CO 2 , H 2 O, O 2 , and N 2 , determine the<br />
mole number of each gas in the products and the airfuel<br />
ratio for this combustion process.<br />
Solution <strong>Chemical</strong> reaction equation:<br />
C 8 H 18 +20 (O 2 + 3.76 N 2 ) xCO 2 + yH 2 O + zO 2 + wN 2<br />
Balance of each element : @ ReactantS = @ ProductS<br />
C: 8 = x x = 8<br />
H: 18 = 2y y = 9<br />
O: (20x2) = 2x +y + 2z z = 7.5<br />
N 2 : 20*3.76 = w w = 75.2<br />
Air Fuel Ratio, AF = m air /m fuel = (NM) air /(NM) fuel<br />
= 24.2 kg air/kg fuel<br />
Theoretical and Actual Combustion<br />
Processes<br />
Complete combustion: 1) all C burns to CO 2<br />
, and 2) all<br />
HC burns to H 2<br />
O<br />
Incomplete combustion: Products contains Unburned<br />
fuel or components สาเหตุหลัก: 1)Insufficient O 2<br />
2)<br />
Insufficient mixing. 3) Dissociation<br />
Stoichiometric Air or Theoretical Air:<br />
• = minimum amount of air needed for complete<br />
combustion.<br />
• no unburned and no O 2<br />
left in products<br />
• = <strong>Chemical</strong>ly correct amount of air or 100%<br />
theoretical air<br />
4