Peripheral B and T cell differentiation - University Institute of ...
Peripheral B and T cell differentiation - University Institute of ...
Peripheral B and T cell differentiation - University Institute of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
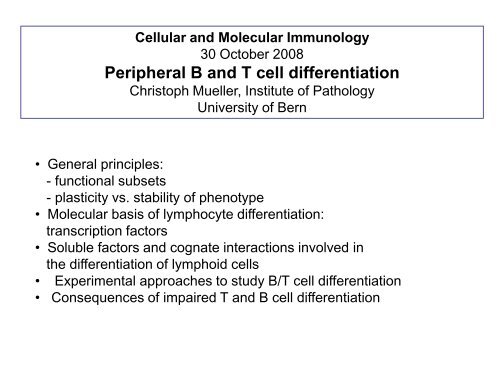
Cellular <strong>and</strong> Molecular Immunology<br />
30 October 2008<br />
<strong>Peripheral</strong> B <strong>and</strong> T <strong>cell</strong> <strong>differentiation</strong><br />
Christoph Mueller, <strong>Institute</strong> <strong>of</strong> Pathology<br />
<strong>University</strong> <strong>of</strong> Bern<br />
• General principles:<br />
- functional subsets<br />
- plasticity vs. stability <strong>of</strong> phenotype<br />
• Molecular basis <strong>of</strong> lymphocyte <strong>differentiation</strong>:<br />
transcription factors<br />
• Soluble factors <strong>and</strong> cognate interactions involved in<br />
the <strong>differentiation</strong> <strong>of</strong> lymphoid <strong>cell</strong>s<br />
• Experimental approaches to study B/T <strong>cell</strong> <strong>differentiation</strong><br />
• Consequences <strong>of</strong> impaired T <strong>and</strong> B <strong>cell</strong> <strong>differentiation</strong>
RAG-1, 2<br />
• RAG1 <strong>and</strong> RAG2 (“Recombination Activation Genes”)<br />
are essential for the rearrangement <strong>of</strong> the Ig <strong>and</strong> TCR<br />
genes<br />
• Mice deficient for either RAG1 <strong>and</strong>/or RAG2 are<br />
deficient for both T <strong>and</strong> B <strong>cell</strong>s (but may still have some<br />
NK <strong>cell</strong>s)<br />
• to prevent the later generation <strong>of</strong> autoreactive T <strong>and</strong> B<br />
<strong>cell</strong>s, the expression <strong>of</strong> these two genes needs to be<br />
tightly regulated
CD8 T <strong>cell</strong> <strong>differentiation</strong>
Functional Heterogeneity <strong>of</strong><br />
CD4 T Lymphocytes<br />
naive CD4 T Cell<br />
Th1 Th0 Th2<br />
ThO: IL2, IL3, IL4, IL5, IL6, IL9, IL10, IFN<br />
Th1: IL2, IFN , TNFlymphotoxin<br />
Th2: IL4, IL5, IL6, IL9, IL10
Functional Heterogeneity<br />
<strong>of</strong> CD4 T Lymphocytes<br />
naïve CD4 T Cells<br />
Th1 Th0 Th2<br />
<strong>cell</strong>ular<br />
immunity<br />
humoral<br />
immunity
Functional Heterogeneity <strong>of</strong> CD4 T<br />
Lymphocytes is Controlled by Different<br />
Transcription Factors<br />
naïve CD4 T Cells<br />
Th1 Th0 Th2<br />
T-bet<br />
GATA-3
Naïve<br />
CD4<br />
IL 4<br />
IL 12<br />
IFN <br />
Th 2 Th 1<br />
Grogan & Locksley Curr Opinion Immunol 14: 366-72; 2002
JCI 109;:431;2002<br />
Pathways thought to regulate<br />
the development <strong>of</strong> Th2 <strong>cell</strong>s
Leprosy<br />
• Chronic - progressive infectious disease, affecting the<br />
skin, peripheral nerves <strong>and</strong> occasionally the respiratory<br />
tract<br />
• Infectious agent: Mycobacterium leprae<br />
• Globally, approx. 10-20 million patients infected,<br />
endemic in tropical areas (e.g. Southeast Asia; India,<br />
South America, Subsaharan Africa)
Leprosy:<br />
different clinical forms <strong>of</strong> the disease<br />
Lepromatous Leprosy:<br />
• Multiple, nodular lesions <strong>of</strong> the skin, in particular, <strong>of</strong> the<br />
face (”lion face").<br />
• Persistent bacteriemia, foamy <strong>cell</strong>-like lesions with<br />
numerous M. leprae present<br />
Tuberculoid Leprosy:<br />
• Singular, small macular lesions <strong>of</strong> the skin.<br />
• <strong>Peripheral</strong> nerves (e.g. N. ulnaris, peronealis, N.<br />
auricularis) are <strong>of</strong>ten affected sensory neuropathy.<br />
• Granuloma are frequent (with only low numbers <strong>of</strong><br />
M. leprae present)
Immunological Spectrum <strong>of</strong> Leprosy<br />
naïve CD4 T <strong>cell</strong>s<br />
Th1 Th0 Th2<br />
<strong>cell</strong>ular<br />
immunity<br />
humoral<br />
immunity<br />
Tuberculoid leprosy<br />
Granuloma formation<br />
Tissue damage may ensue<br />
Lepromatous leprosy<br />
Persistence <strong>of</strong> M. leprae<br />
Disfiguring disorder
Type IV Hypersensitivity reactions<br />
Fig. 5-11<br />
Kumar 6th edition
Pathogens may influence the resulting adaptive immune response<br />
Science 302: 993-4; 2003
Figure 1 Stimulating the Th1 or Th2 response. In both pathways, dendritic <strong>cell</strong>s internalize the<br />
pathogen. They present its antigens to T <strong>cell</strong>s, which recognize antigens through their T-<strong>cell</strong> receptors<br />
(TCR). a, Organisms such as intra<strong>cell</strong>ular bacteria or viruses are recognized by the Toll-like<br />
receptors on dendritic <strong>cell</strong>s; the resulting signals induce the secretion <strong>of</strong> interleukin-12 (IL-12) <strong>and</strong><br />
<strong>differentiation</strong> <strong>of</strong> CD4 T <strong>cell</strong>s into the Th1 lineage that produces gamma interferon (IFN-). b, How<br />
dendritic <strong>cell</strong>s recognize larger pathogens, such as parasitic worms, is not known. But the end result<br />
is <strong>differentiation</strong> <strong>of</strong> Th2 effector <strong>cell</strong>s regulated by T-<strong>cell</strong>-produced interleukin-4 (IL-4).<br />
Information1, 2 on the link between dendritic <strong>cell</strong>s <strong>and</strong> T <strong>cell</strong>s suggests that the former express<br />
different Notch lig<strong>and</strong>s — Delta or Jagged — under different conditions. Jagged is specifically<br />
induced by stimuli known to induce Th2 <strong>differentiation</strong>. Notch signals (Notch-IC) can induce<br />
transcription <strong>of</strong> IL-4 through direct binding <strong>of</strong> RBPJ to the IL-4 promoter1<br />
Nature 430, 150 - 151 (08 July 2004)
# Publications per Year (PubMed)<br />
Publications on Suppressor T <strong>cell</strong>s <strong>and</strong><br />
Regulatory T <strong>cell</strong>s<br />
300<br />
250<br />
200<br />
Suppressor T <strong>cell</strong>s<br />
Regulatory T <strong>cell</strong>s<br />
150<br />
100<br />
50<br />
0
Rregulatory T <strong>cell</strong> subsets<br />
Natural regulatory T <strong>cell</strong>s express the <strong>cell</strong>-surface marker CD25 <strong>and</strong> the<br />
transcriptional repressor FOXP3 (forkhead box P3). These <strong>cell</strong>s mature <strong>and</strong> migrate<br />
from the thymus <strong>and</strong> constitute 5–10% <strong>of</strong> peripheral T <strong>cell</strong>s in normal mice. Other<br />
populations <strong>of</strong> antigen-specific regulatory T <strong>cell</strong>s can be induced from naive<br />
CD4 + CD25 - or CD8 + CD25 - T <strong>cell</strong>s in the periphery under the influence <strong>of</strong> semimature<br />
dendritic <strong>cell</strong>s, interleukin-10 (IL-10), transforming growth factor- (TGF-) <strong>and</strong><br />
possibly interferon- (IFN-). The inducible populations <strong>of</strong> regulatory T <strong>cell</strong>s include<br />
distinct subtypes <strong>of</strong> CD4 + T <strong>cell</strong>: T regulatory 1 (T R 1) <strong>cell</strong>s, which secrete high levels<br />
<strong>of</strong> IL-10, no IL-4 <strong>and</strong> no or low levels <strong>of</strong> IFN-; <strong>and</strong> T helper 3 (T H 3) <strong>cell</strong>s, which<br />
secrete high levels <strong>of</strong> TGF-. Although CD8 + T <strong>cell</strong>s are normally associated with<br />
cytotoxic T-lymphocyte function <strong>and</strong> IFN- production, these <strong>cell</strong>s or a subtype <strong>of</strong><br />
these <strong>cell</strong>s can secrete IL-10 <strong>and</strong> have been called CD8 + regulatory T <strong>cell</strong>s.
IFN-mediated STAT1 signaling leads to the induction <strong>of</strong> T-bet <strong>and</strong><br />
<strong>differentiation</strong> <strong>of</strong> T H 1 <strong>cell</strong>s. IFN- production is further potentiated by<br />
inflammatory cytokines such as IL-6. TGFß- induces Foxp3 expression in naive<br />
CD4 + T <strong>cell</strong>s <strong>and</strong> their <strong>differentiation</strong> into induced T reg <strong>cell</strong>s. IL-6 is a potent<br />
inhibitor <strong>of</strong> TGFß--induced Foxp3 induction in CD4 + T <strong>cell</strong>s. However,<br />
stimulation with both TGFß- <strong>and</strong> IL-6 results in the expression <strong>of</strong> RORt <strong>and</strong><br />
subsequent <strong>differentiation</strong> <strong>of</strong> T H -17 <strong>cell</strong>s.
Natural regulatory T <strong>cell</strong>s express the <strong>cell</strong>-surface marker CD25 <strong>and</strong> the<br />
transcriptional repressor FOXP3 (forkhead box P3). These <strong>cell</strong>s mature <strong>and</strong> migrate<br />
from the thymus <strong>and</strong> constitute 5–10% <strong>of</strong> peripheral T <strong>cell</strong>s in normal mice. Other<br />
populations <strong>of</strong> antigen-specific regulatory T <strong>cell</strong>s can be induced from naive<br />
CD4 + CD25 - or CD8 + CD25 - T <strong>cell</strong>s in the periphery under the influence <strong>of</strong> semimature<br />
dendritic <strong>cell</strong>s, interleukin-10 (IL-10), transforming growth factor- (TGF-)<br />
<strong>and</strong> possibly interferon- (IFN-). The inducible populations <strong>of</strong> regulatory T <strong>cell</strong>s<br />
include distinct subtypes <strong>of</strong> CD4 + T <strong>cell</strong>: T regulatory 1 (T R 1) <strong>cell</strong>s, which secrete<br />
high levels <strong>of</strong> IL-10, no IL-4 <strong>and</strong> no or low levels <strong>of</strong> IFN-; <strong>and</strong> T helper 3 (T H 3) <strong>cell</strong>s,<br />
which secrete high levels <strong>of</strong> TGF-. Although CD8 + T <strong>cell</strong>s are normally associated<br />
with cytotoxic T-lymphocyte function <strong>and</strong> IFN- production, these <strong>cell</strong>s or a subtype<br />
<strong>of</strong> these <strong>cell</strong>s can secrete IL-10 <strong>and</strong> have been called CD8 + regulatory T <strong>cell</strong>s.<br />
SL Reiner Cell 129: 33-36; 2007
Mechanism(s) <strong>of</strong> suppression.<br />
Various molecular <strong>and</strong> <strong>cell</strong>ular<br />
events have been described to<br />
explain how Treg can suppress<br />
immune responses. They<br />
include: IL-2 gene expression<br />
inhibition, modulation <strong>of</strong><br />
costimulatory molecules on APCs<br />
<strong>and</strong> interaction <strong>of</strong> LAG3 with<br />
MHC class II molecules (a),<br />
immunosuppressive cytokine<br />
secretion (b), induction <strong>of</strong><br />
tryptophan catabolism through<br />
CTLA-4 (c) <strong>and</strong> cytotoxicity (d).<br />
However, none <strong>of</strong> those<br />
mechanisms can explain all<br />
aspects <strong>of</strong> suppression. It is<br />
probable that various<br />
combinations <strong>of</strong> several<br />
mechanisms are operating,<br />
depending on the milieu <strong>and</strong> the<br />
type <strong>of</strong> immune responses. It is<br />
also possible that there might be<br />
a single key mechanism that has<br />
not been found yet (e).<br />
Abbreviations: APC, antigen<br />
presenting <strong>cell</strong>; TCR, T <strong>cell</strong><br />
receptor.
B <strong>cell</strong>s ….
CD4 T-Zelle<br />
CD40L<br />
T-Zell-Hilfe<br />
durch Zytokine<br />
2.Signal:<br />
Quervernetzung der<br />
Ig durch Antigen oder<br />
Aktivierung durch CD40L<br />
CD40<br />
1. Signal:<br />
Bindung des Antigen<br />
an Ig<br />
2. Signal<br />
naive B - Zelle<br />
kein<br />
2. Signal<br />
B-Gedächtniszelle