INNOVA E3 - Scican.uk.com
INNOVA E3 - Scican.uk.com
INNOVA E3 - Scican.uk.com
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
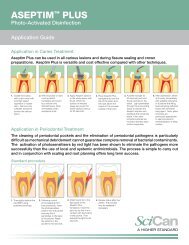
Perfect Match of Reprocessing<br />
Parameters at Maximum Flexibility<br />
R e p r o c e s s i n g P r o c e s s a n d Te c h n o l o g y<br />
BHT – the expert for innovative<br />
cleaning and disinfection<br />
technology for the reprocessing<br />
of endoscopes<br />
For more than 25 years BHT has<br />
been one of the acknowledged leaders<br />
in the development and production of<br />
innovative washer-disinfectors in the<br />
areas of healthcare, laboratory and<br />
pharmaceutical industry. BHT is a<br />
medium sized , internationally active<br />
enterprise with 2 production sites in<br />
the heart of Bavaria, Germany.<br />
Frost & Sullivan appreciated the<br />
innovative power, specifically in the<br />
field of endoscopy reprocessing by<br />
awarding the Product Innovation<br />
Price Healthcare 2005 for the BHT<br />
<strong>INNOVA</strong>® CC for checking the individual<br />
channels of flexible endoscopes.<br />
Extensive patents and other protective<br />
rights document the high potential<br />
of BHT. Of course, BHT is certified<br />
according to ISO 9001:2000 and<br />
EN 13485:2003.<br />
The technology of endoscopy has open ed<br />
new avenidas in healthcare for diagno sis<br />
and treatment. At the same time it has<br />
created new risks: Endoscopes can be<br />
a dangerous sources of infections. The<br />
cleaning and disinfection of these sensitive<br />
devices requires a <strong>com</strong>prehensive<br />
know-how of the manufacturer of washer-disinfectors<br />
(WDs). BHT has proven<br />
its <strong>com</strong>petence by introducing the first<br />
fully automatic WD already in 1988. Since<br />
that time BHT is actively engaged to<br />
improve the safety conditions in reprocessing<br />
of medical devices, e.g. within<br />
the <strong>com</strong>mittee for preparing the new<br />
standard<br />
prEN ISO 15883 -1/5.<br />
In all BHT WDs parameters such as time,<br />
temperature and used chemicals are<br />
precisely adjusted to the medical device<br />
to be reprocessed. BHT has realized a<br />
validatable reprocessing process where<br />
all phases are supervised.<br />
1. Reliable sensors monitor the process<br />
data important for the result of the<br />
process.<br />
2 . The micro<strong>com</strong>puter supervises and<br />
registers all parameters.<br />
3. Patented cleaning mechanics and<br />
process technology ensures superior<br />
efficiency and hygienic safety.<br />
4. Patented, electronically supervised<br />
volume dosing guarantees the optimal<br />
employment of chemicals.<br />
Air-/water channel<br />
Suction channel Operation part Biopsy channel Distal end<br />
Exit to<br />
insertion tube<br />
Water-/<br />
disinfectant<br />
supply<br />
Supply plug<br />
Channel for rinse of lens<br />
Additional channel for<br />
rinse of biopsy channel<br />
Suction area<br />
Supply tube<br />
Suction channel<br />
CO2-channel<br />
Innova ®<br />
New <strong>E3</strong> Series