The Geometry The Nucleus
The Geometry The Nucleus
The Geometry The Nucleus
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
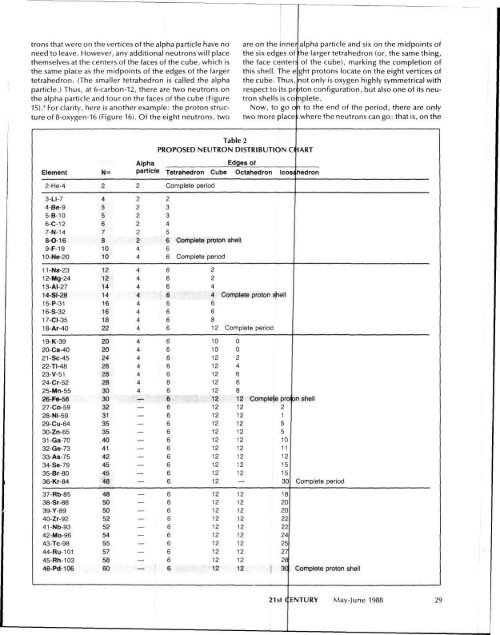
trons that were on the vertices of the alpha particle have no<br />
need to leave. However, any additional neutrons will place<br />
themselves at the centers of the faces of the cube, which is<br />
the same place as the midpoints of the edges of the larger<br />
tetrahedron. (<strong>The</strong> smaller tetrahedron is called the alpha<br />
particle.) Thus, at 6-carbon-12, there are two neutrons on<br />
the alpha particle and four on the faces of the cube (Figure<br />
15)." For clarity, here is another example: the proton structure<br />
of 8-oxygen-16 (Figure 16). Of the eight neutrons, two<br />
are on the inner alpha particle and six on the midpoints of<br />
the six edges of :he larger tetrahedron (or, the same thing,<br />
the face center; of the cube), marking the completion of<br />
this shell. <strong>The</strong> e ght protons locate on the eight vertices of<br />
the cube. Thus, not only is oxygen highly symmetrical with<br />
respect to its pr )ton configuration, but also one of its neutron<br />
shells is co nplete.<br />
Now, to go o i to the end of the period, there are only<br />
two more place ; where the neutrons can go: that is, on the<br />
Element<br />
2-He-4<br />
3-Li-7<br />
4-Be-9<br />
5-B-10<br />
6-C-12<br />
7-N-14<br />
8-0-16<br />
9-F-19<br />
10-Ne-20<br />
11-Na-23<br />
12-Mg-24<br />
13-AI-27<br />
14-SI-28<br />
15-P-31<br />
16-S-32<br />
17-CI-35<br />
18-Ar-40<br />
19-K-39<br />
20-Ca-40<br />
21-SC-45<br />
22-TI-48<br />
23-V-51<br />
24-Cr-52<br />
25-Mn-55<br />
26-Fe-56<br />
27-CO-59<br />
28-NI-59<br />
29-CU-64<br />
30-Zn-65<br />
31-Ga-70<br />
32-Ge-73<br />
33-A8-75<br />
34-Se-79<br />
35-Br-80<br />
36-Kr-84<br />
37-Rb-85<br />
38-Sr-88<br />
39-Y-89<br />
40-Zr-92<br />
41-Nb-93<br />
42-MO-96<br />
43-TC-98<br />
44-RU-101<br />
45-Rh-103<br />
46-Pd-106<br />
N=<br />
2<br />
4<br />
5<br />
5<br />
12<br />
12<br />
14<br />
14<br />
16<br />
16<br />
18<br />
22<br />
20<br />
20<br />
24<br />
26<br />
28<br />
28<br />
30<br />
30<br />
32<br />
31<br />
35<br />
35<br />
40<br />
41<br />
42<br />
45<br />
45<br />
48<br />
48<br />
50<br />
50<br />
52<br />
52<br />
54<br />
55<br />
57<br />
58<br />
60<br />
Alpha<br />
particle<br />
Table 2<br />
PROPOSED NEUTRON DISTRIBUTION C*IART<br />
Edges of<br />
Tetrahedron Cube Octahedron Icos: hedron<br />
2 Complete period<br />
2 2<br />
2 3<br />
2<br />
2<br />
2<br />
3<br />
4<br />
5<br />
2 6 Complete proton shell<br />
4 T6<br />
4 6 Complete period<br />
4 6 2<br />
4 6 2<br />
4 6 4 [<br />
4 6 4 Complete proton shell<br />
4 6 6<br />
4 6 6<br />
4 6 8<br />
4 6 12 Complete period<br />
4 6 10 0<br />
4 6 10 0<br />
4 6 12 2<br />
4 6 12 4<br />
4 6 12 6<br />
4 6 12 6<br />
4 6 12 8 J<br />
— 6 12 12 Complete pro on shell<br />
— 6 12 12 2<br />
— 6 12 12 1<br />
— 6 12 12 5<br />
— 6 12 12 5<br />
— 6 12 12 10<br />
— 6 12 12 11<br />
— 6 12 12 12<br />
— 6 12 12 15<br />
— 6 12 12 15<br />
— 6 12 — 30 Complete period<br />
6 12 12 18<br />
— 6 12 12 20<br />
— 6 12 12 20<br />
— 6 12 12 22<br />
— 6 12 12 22<br />
— 6 12 12 24<br />
— 6 12 12 25<br />
— 6 12 12 27<br />
— 6 12 12 28<br />
— 6 12 12 3C<br />
Complete proton shell<br />
21st CENTURY May-June 1988