N-linked Glycan Analysis using cHiPLC® System - Eksigent
N-linked Glycan Analysis using cHiPLC® System - Eksigent
N-linked Glycan Analysis using cHiPLC® System - Eksigent
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
N-<strong>linked</strong> <strong>Glycan</strong> <strong>Analysis</strong> <strong>using</strong> cHiPLC ® <strong>System</strong><br />
Xiang Zhu 1 , Justin Hyche 2 , Remco van Soest 1<br />
1 <strong>Eksigent</strong>, part of AB SCIEX, Dublin, CA; 2 ProZyme, Hayward, CA<br />
Glycosylation is known as one of the most important protein<br />
post-translational modifications in eukaryotic cells. <strong>Glycan</strong>s are<br />
involved in a number of biological processes such as cell<br />
adhesion, immune recognition, and protein regulation. Abnormal<br />
glycosylation has been observed in many types of cancers. One<br />
of the most commonly emergent changes in glycosylation<br />
observed in cancer cells is the increasing branching on complex<br />
N-<strong>linked</strong> glycans. Therefore, understanding the changes in<br />
glycan profiles on proteins has become very important for<br />
biomarker studies. LC-MS has been recognized as a useful<br />
technique to qualitatively and quantitatively analyze glycan<br />
structures.<br />
The separation and analysis of glycoprotein released glycans<br />
<strong>using</strong> reversed phase LC-MS is always a challenging task<br />
because these molecules are usually very hydrophilic, difficult to<br />
ionize and contain a variety of complex compositions, branching<br />
and linkage isomers. Herein, a chip based nanoLC system, the<br />
cHiPLC ® system 1 , was used to separate and identify N-<strong>linked</strong><br />
glycans from some standard glycoproteins. The glycan<br />
separation utility of both hydrophilic interaction chromatography<br />
(HILIC) and porous graphitic carbon (PGC) chip columns was<br />
investigated.<br />
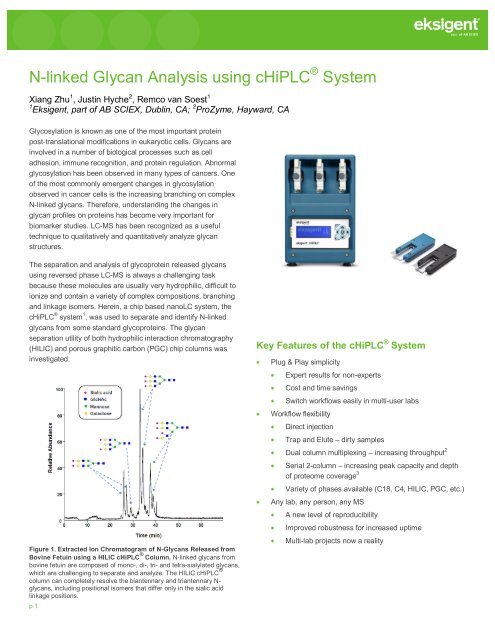
Figure 1. Extracted Ion Chromatogram of N-<strong>Glycan</strong>s Released from<br />
Bovine Fetuin <strong>using</strong> a HILIC cHiPLC ® Column. N-<strong>linked</strong> glycans from<br />
bovine fetuin are composed of mono-, di-, tri- and tetra-sialylated glycans,<br />
which are challenging to separate and analyze. The HILIC cHiPLC ®<br />
column can completely resolve the biantennary and triantennary N-<br />
glycans, including positional isomers that differ only in the sialic acid<br />
linkage positions.<br />
p 1<br />
Key Features of the cHiPLC ® <strong>System</strong><br />
Plug & Play simplicity<br />
Expert results for non-experts<br />
Cost and time savings<br />
Switch workflows easily in multi-user labs<br />
Workflow flexibility<br />
Direct injection<br />
Trap and Elute – dirty samples<br />
Dual column multiplexing – increasing throughput 2<br />
Serial 2-column – increasing peak capacity and depth<br />
of proteome coverage 3<br />
Variety of phases available (C18, C4, HILIC, PGC, etc.)<br />
Any lab, any person, any MS<br />
A new level of reproducibility<br />
Improved robustness for increased uptime<br />
Multi-lab projects now a reality
Experimental<br />
Sample Preparation: Underivatized N-glycan mixtures from<br />
bovine fetuin and human immunoglobulin G (IgG) (ProZyme,<br />
Hayward, CA) were used to test the two separation phases.<br />
Samples were freshly dissolved in 20% mobile phase A/80%<br />
mobile phase B solution for HILIC analysis and 100% mobile<br />
phase A for PGC separation.<br />
LC-MS/MS: An <strong>Eksigent</strong> NanoLC-Ultra ® 1D plus system<br />
(<strong>Eksigent</strong>, part of AB SCIEX Dublin, CA, USA) was used in<br />
combination with a cHiPLC ® system (<strong>Eksigent</strong>) in direct inject<br />
mode. The NanoLC-Ultra ® system delivers the solvent based on<br />
binary gradient pumps that use patented Microfluidic Flow<br />
Control (MFC) pump technology. The porous graphitic carbon<br />
(PGC) cHiPLC ® column was packed with 3 µm graphitized<br />
carbon. The hydrophilic interaction chromatography (HILIC)<br />
cHiPLC ® column contained 2.7 µm HALO Fused-Core particles<br />
as packing material (Advanced Materials Technology,<br />
Wilmington, DE, USA). The MS data was acquired <strong>using</strong> an LTQ<br />
ion trap mass spectrometer (Thermo Fisher, San Jose, CA,<br />
USA). Detailed experimental conditions were listed in Table 1.<br />
<strong>Glycan</strong> Separation Comparisons<br />
<strong>Glycan</strong>s are typically hydrophilic and thus not retained well by<br />
reversed phase liquid chromatography. Common alternative<br />
column phases include porous graphitic carbon (PGC) and<br />
hydrophilic interaction chromatography (HILIC). Both of these<br />
column phases retain hydrophilic compounds more than typical<br />
reversed phase chromatography and result in longer retention<br />
times vs. reversed phase LC. Therefore PGC and HILIC columns<br />
are often applied for resolving complex glycan mixtures.<br />
Figure 2. Extracted Ion Chromatogram of N-<strong>Glycan</strong>s Released from<br />
Bovine Fetuin <strong>using</strong> cHiPLC ® PGC Column. Compared to C18 phase,<br />
the PGC column retains the hydrophilic molecules much better and<br />
separates the positional isomers as well. The tetra-sialylated glycans are<br />
not separated as well as with the HILIC column (Figure 1), possibly due<br />
to their strong interaction with the PGC stationary phase.<br />
Figure 1 shows an example of <strong>using</strong> a HILIC-nanoLC/MS<br />
approach for the separation and detection of N-<strong>linked</strong> glycans<br />
from bovine fetuin. This particular sample contains a mixture of<br />
mono-, di-, tri-, and tetra-sialylated glycans, making the<br />
separation very challenging. Separation and identification of<br />
these N-glycans was also performed <strong>using</strong> a PGC cHiPLC ®<br />
column (Figure 2). One of the advantages of <strong>using</strong> PGC<br />
compared to HILIC is that the sample can be dissolved in high<br />
aqueous solutions to reduce sample loss. Bi-antennary and triantennary<br />
structures are well separated including the isomeric<br />
species. Compared to the HILIC cHiPLC column, the tetrasialylated<br />
glycans give broader peaks <strong>using</strong> the PGC column,<br />
Table 1. LC-MS Conditions for N-<strong>Glycan</strong> <strong>Analysis</strong>.<br />
HILIC Fetuin N-glycan PGC Fetuin N-glycan HILIC IgG N-glycan PGC IgG N-glycan<br />
Sample Concentration 2 ng/µL 2 ng/µL 20 ng/µL 2 ng/µL<br />
Injection Volume 1 µL 1 µL 1 µL 1 µL<br />
Column<br />
Halo HILIC cHiPLC ®<br />
75 µm x 15 cm<br />
PGC cHiPLC ®<br />
75 µm x 15 cm<br />
Halo HILIC cHiPLC ®<br />
75 µm x 15 cm<br />
PGC cHiPLC ®<br />
75 µm x 15 cm<br />
Mobile Phase A<br />
50mM ammonium formate<br />
pH 4.0<br />
0.08% ammonia<br />
pH 10.0<br />
50mM ammonium formate<br />
pH 4.0<br />
Water (0.1% formic acid)<br />
pH 4.0<br />
Mobile Phase B<br />
Acetonitrile<br />
(0.1% formic acid)<br />
Acetonitrile<br />
(0.1% formic acid)<br />
Acetonitrile<br />
(0.1% formic acid)<br />
Acetonitrile<br />
(0.1% formic acid)<br />
Temperature 60 °C 60 °C 60 °C 60 °C<br />
Detection LTQ, negative mode LTQ, negative mode LTQ, positive mode LTQ, positive mode<br />
Flowrate 300 nL/min 300 nL/min 300 nL/min 300 nL/min<br />
Gradient 70-60% B in 60 min 5-50% B in 30 min 90-60% B in 40 min 3-40% B in 40 min<br />
p 2
The detection <strong>using</strong> the HILIC cHiPLC ® column also identified<br />
most of the major IgG N-glycans, as shown in Figure 5. Some<br />
glycans were only detected either in HILIC or PGC conditions,<br />
making the two methods complementary to each other.<br />
Figure 3. Identified N-<strong>linked</strong> <strong>Glycan</strong>s Structures from IgG. Most of<br />
these glycans were identified <strong>using</strong> both cHiPLC ® columns. Some were<br />
detected either in HILIC or PGC LC-MS only as indicated by the numbers<br />
labeling the peaks in Figures 4 and 5.<br />
possibly due to stronger interaction with the PGC stationary<br />
phases.<br />
As shown in Figure 4, a mixture of high mannose, complex and<br />
hybrid N-<strong>linked</strong> glycans released from human IgG were well<br />
separated <strong>using</strong> PGC cHiPLC ® column. Different isomers were<br />
resolved as well and shown as two separated peaks.<br />
Figure 5. Extracted Ion Chromatogram of Human IgG N-<strong>Glycan</strong>s<br />
<strong>using</strong> HILIC cHiPLC ® Column. Some glycans were only identified in<br />
this method, making it complementary to PGC analysis.<br />
Conclusions<br />
Two nanoflow cHiPLC ® columns (HILIC and PGC) were coupled<br />
with a high performance NanoLC-Ultra ® system and utilized for<br />
the analysis of hydrophilic carbohydrates. Both methods<br />
separated complex mixtures of N-glycans including some<br />
isomeric species, enabling direct identification of the glycans by<br />
LC/MS.<br />
References<br />
1. cHiPLC ® -nanoflex <strong>System</strong>: Making nanoLC/MS Easier and<br />
More Reproducible. <strong>Eksigent</strong> Product Note 0910-1000<br />
Figure 4. Extracted Ion Chromatogram of Human IgG N-<strong>Glycan</strong>s<br />
<strong>using</strong> PGC cHiPLC ® Column. The peak number and corresponding<br />
structures were listed in Figure 3. A combination of high mannose,<br />
complex and hybrid N-glycans were separated <strong>using</strong> graphitized carbon<br />
chip column at nanoflow conditions. Different isomers were also<br />
resolved and split into two peaks.<br />
2. Increasing Throughput of nanoLC <strong>using</strong> Two-Column<br />
Switching Workflows. AB SCIEX Technical Note 1870411-<br />
01<br />
3. Increasing Depth of Coverage <strong>using</strong> Serial Two-Column<br />
Workflows. AB SCIEX Technical Note 1870211-02<br />
For Research Use Only. Not for use in diagnostic procedures.<br />
© 2012 <strong>Eksigent</strong>, a division of AB SCIEX. The trademarks mentioned herein are the property of AB Sciex Pte. Ltd. or their respective owners. AB SCIEX TM is being used under license. All<br />
rights reserved. Information subject to change without notice.<br />
Patents pending worldwide. Printed in the USA. 6540212-01<br />
p 3