Debunking Human Health Risk, APPENDIX D - LBAMspray.com
Debunking Human Health Risk, APPENDIX D - LBAMspray.com
Debunking Human Health Risk, APPENDIX D - LBAMspray.com
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
LIGHT BROWN APPLE MOTH ERADICATION PROJECT<br />
<strong>APPENDIX</strong> D<br />
DRAFT PEIR<br />
HUMAN HEALTH RISK ASSESSMENT<br />
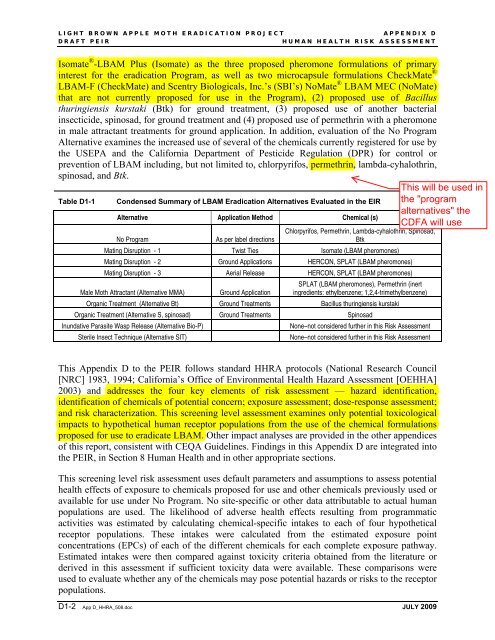
Isomate ® -LBAM Plus (Isomate) as the three proposed pheromone formulations of primary<br />
interest for the eradication Program, as well as two microcapsule formulations CheckMate ®<br />
LBAM-F (CheckMate) and Scentry Biologicals, Inc.’s (SBI’s) NoMate ® LBAM MEC (NoMate)<br />
that are not currently proposed for use in the Program), (2) proposed use of Bacillus<br />
thuringiensis kurstaki (Btk) for ground treatment, (3) proposed use of another bacterial<br />
insecticide, spinosad, for ground treatment and (4) proposed use of permethrin with a pheromone<br />
in male attractant treatments for ground application. In addition, evaluation of the No Program<br />
Alternative examines the increased use of several of the chemicals currently registered for use by<br />
the USEPA and the California Department of Pesticide Regulation (DPR) for control or<br />
prevention of LBAM including, but not limited to, chlorpyrifos, permethrin, lambda-cyhalothrin,<br />
spinosad, and Btk.<br />
Table D1-1<br />
Condensed Summary of LBAM Eradication Alternatives Evaluated in the EIR<br />
Alternative Application Method Chemical (s)<br />
No Program<br />
As per label directions<br />
Chlorpyrifos, Permethrin, Lambda-cyhalothrin, Spinosad,<br />
Btk<br />
Mating Disruption - 1 Twist Ties Isomate (LBAM pheromones)<br />
Mating Disruption - 2 Ground Applications HERCON, SPLAT (LBAM pheromones)<br />
Mating Disruption - 3 Aerial Release HERCON, SPLAT (LBAM pheromones)<br />
Male Moth Attractant (Alternative MMA)<br />
Ground Application<br />
SPLAT (LBAM pheromones), Permethrin (inert<br />
ingredients: ethylbenzene; 1,2,4-trimethylbenzene)<br />
Organic Treatment (Alternative Bt) Ground Treatments Bacillus thuringiensis kurstaki<br />
Organic Treatment (Alternative S, spinosad) Ground Treatments Spinosad<br />
Inundative Parasite Wasp Release (Alternative Bio-P)<br />
Sterile Insect Technique (Alternative SIT)<br />
None–not considered further in this <strong>Risk</strong> Assessment<br />
None–not considered further in this <strong>Risk</strong> Assessment<br />
This Appendix D to the PEIR follows standard HHRA protocols (National Research Council<br />
[NRC] 1983, 1994; California’s Office of Environmental <strong>Health</strong> Hazard Assessment [OEHHA]<br />
2003) and addresses the four key elements of risk assessment — hazard identification,<br />
identification of chemicals of potential concern; exposure assessment; dose-response assessment;<br />
and risk characterization. This screening level assessment examines only potential toxicological<br />
impacts to hypothetical human receptor populations from the use of the chemical formulations<br />
proposed for use to eradicate LBAM. Other impact analyses are provided in the other appendices<br />
of this report, consistent with CEQA Guidelines. Findings in this Appendix D are integrated into<br />
the PEIR, in Section 8 <strong>Human</strong> <strong>Health</strong> and in other appropriate sections.<br />
This screening level risk assessment uses default parameters and assumptions to assess potential<br />
health effects of exposure to chemicals proposed for use and other chemicals previously used or<br />
available for use under No Program. No site-specific or other data attributable to actual human<br />
populations are used. The likelihood of adverse health effects resulting from programmatic<br />
activities was estimated by calculating chemical-specific intakes to each of four hypothetical<br />
receptor populations. These intakes were calculated from the estimated exposure point<br />
concentrations (EPCs) of each of the different chemicals for each <strong>com</strong>plete exposure pathway.<br />
Estimated intakes were then <strong>com</strong>pared against toxicity criteria obtained from the literature or<br />
derived in this assessment if sufficient toxicity data were available. These <strong>com</strong>parisons were<br />
used to evaluate whether any of the chemicals may pose potential hazards or risks to the receptor<br />
populations.<br />
D1-2 App D_HHRA_508.doc JULY 2009