Debunking Human Health Risk, APPENDIX D - LBAMspray.com
Debunking Human Health Risk, APPENDIX D - LBAMspray.com
Debunking Human Health Risk, APPENDIX D - LBAMspray.com
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
SECTION D3<br />
TOXICITY ASSESSMENT<br />
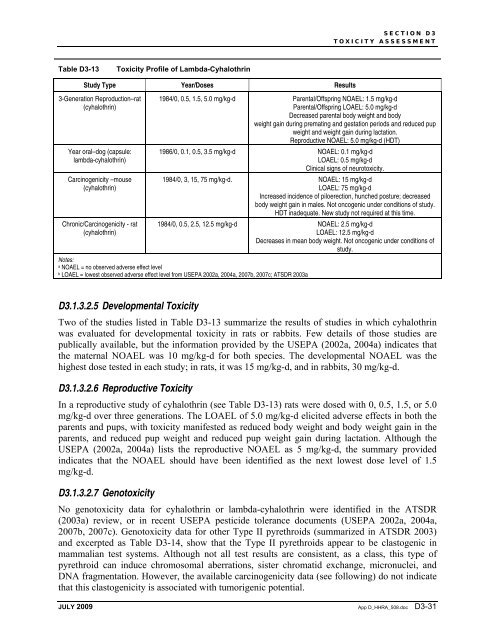
Table D3-13<br />
Toxicity Profile of Lambda-Cyhalothrin<br />
Study Type Year/Doses Results<br />
3-Generation Reproduction–rat<br />
(cyhalothrin)<br />
Year oral–dog (capsule:<br />
lambda-cyhalothrin)<br />
Carcinogenicity –mouse<br />
(cyhalothrin)<br />
Chronic/Carcinogenicity - rat<br />
(cyhalothrin)<br />
1984/0, 0.5, 1.5, 5.0 mg/kg-d Parental/Offspring NOAEL: 1.5 mg/kg-d<br />
Parental/Offspring LOAEL: 5.0 mg/kg-d<br />
Decreased parental body weight and body<br />
weight gain during premating and gestation periods and reduced pup<br />
weight and weight gain during lactation.<br />
Reproductive NOAEL: 5.0 mg/kg-d (HDT)<br />
1986/0, 0.1, 0.5, 3.5 mg/kg-d NOAEL: 0.1 mg/kg-d<br />
LOAEL: 0.5 mg/kg-d<br />
Clinical signs of neurotoxicity.<br />
1984/0, 3, 15, 75 mg/kg-d. NOAEL: 15 mg/kg-d<br />
LOAEL: 75 mg/kg-d<br />
Increased incidence of piloerection, hunched posture; decreased<br />
body weight gain in males. Not oncogenic under conditions of study.<br />
HDT inadequate. New study not required at this time.<br />
1984/0, 0.5, 2.5, 12.5 mg/kg-d NOAEL: 2.5 mg/kg-d<br />
LOAEL: 12.5 mg/kg-d<br />
Decreases in mean body weight. Not oncogenic under conditions of<br />
study.<br />
Notes:<br />
a NOAEL = no observed adverse effect level<br />
b LOAEL = lowest observed adverse effect level from USEPA 2002a, 2004a, 2007b, 2007c; ATSDR 2003a<br />
D3.1.3.2.5 Developmental Toxicity<br />
Two of the studies listed in Table D3-13 summarize the results of studies in which cyhalothrin<br />
was evaluated for developmental toxicity in rats or rabbits. Few details of those studies are<br />
publically available, but the information provided by the USEPA (2002a, 2004a) indicates that<br />
the maternal NOAEL was 10 mg/kg-d for both species. The developmental NOAEL was the<br />
highest dose tested in each study; in rats, it was 15 mg/kg-d, and in rabbits, 30 mg/kg-d.<br />
D3.1.3.2.6 Reproductive Toxicity<br />
In a reproductive study of cyhalothrin (see Table D3-13) rats were dosed with 0, 0.5, 1.5, or 5.0<br />
mg/kg-d over three generations. The LOAEL of 5.0 mg/kg-d elicited adverse effects in both the<br />
parents and pups, with toxicity manifested as reduced body weight and body weight gain in the<br />
parents, and reduced pup weight and reduced pup weight gain during lactation. Although the<br />
USEPA (2002a, 2004a) lists the reproductive NOAEL as 5 mg/kg-d, the summary provided<br />
indicates that the NOAEL should have been identified as the next lowest dose level of 1.5<br />
mg/kg-d.<br />
D3.1.3.2.7 Genotoxicity<br />
No genotoxicity data for cyhalothrin or lambda-cyhalothrin were identified in the ATSDR<br />
(2003a) review, or in recent USEPA pesticide tolerance documents (USEPA 2002a, 2004a,<br />
2007b, 2007c). Genotoxicity data for other Type II pyrethroids (summarized in ATSDR 2003)<br />
and excerpted as Table D3-14, show that the Type II pyrethroids appear to be clastogenic in<br />
mammalian test systems. Although not all test results are consistent, as a class, this type of<br />
pyrethroid can induce chromosomal aberrations, sister chromatid exchange, micronuclei, and<br />
DNA fragmentation. However, the available carcinogenicity data (see following) do not indicate<br />
that this clastogenicity is associated with tumorigenic potential.<br />
JULY 2009 App D_HHRA_508.doc D3-31