Debunking Human Health Risk, APPENDIX D - LBAMspray.com
Debunking Human Health Risk, APPENDIX D - LBAMspray.com
Debunking Human Health Risk, APPENDIX D - LBAMspray.com
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
LIGHT BROWN APPLE MOTH ERADICATION PROJECT<br />
<strong>APPENDIX</strong> D<br />
DRAFT PEIR<br />
HUMAN HEALTH RISK ASSESSMENT<br />
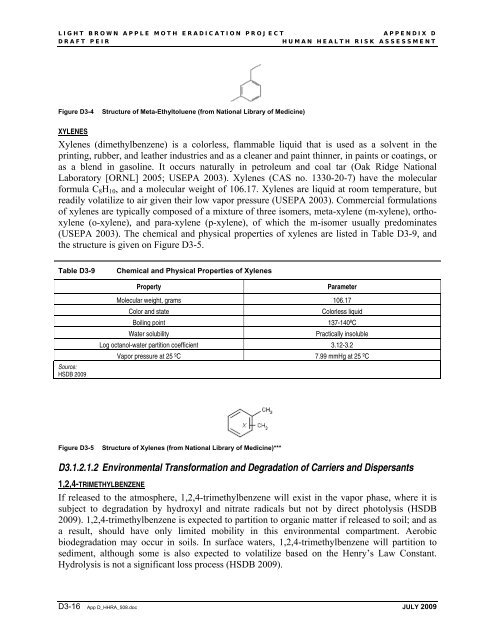
Figure D3-4<br />
Structure of Meta-Ethyltoluene (from National Library of Medicine)<br />
XYLENES<br />
Xylenes (dimethylbenzene) is a colorless, flammable liquid that is used as a solvent in the<br />
printing, rubber, and leather industries and as a cleaner and paint thinner, in paints or coatings, or<br />
as a blend in gasoline. It occurs naturally in petroleum and coal tar (Oak Ridge National<br />
Laboratory [ORNL] 2005; USEPA 2003). Xylenes (CAS no. 1330-20-7) have the molecular<br />
formula C 8 H 10 , and a molecular weight of 106.17. Xylenes are liquid at room temperature, but<br />
readily volatilize to air given their low vapor pressure (USEPA 2003). Commercial formulations<br />
of xylenes are typically <strong>com</strong>posed of a mixture of three isomers, meta-xylene (m-xylene), orthoxylene<br />
(o-xylene), and para-xylene (p-xylene), of which the m-isomer usually predominates<br />
(USEPA 2003). The chemical and physical properties of xylenes are listed in Table D3-9, and<br />
the structure is given on Figure D3-5.<br />
Table D3-9<br />
Chemical and Physical Properties of Xylenes<br />
Property<br />
Parameter<br />
Source:<br />
HSDB 2009<br />
Molecular weight, grams 106.17<br />
Color and state<br />
Colorless liquid<br />
Boiling point<br />
137-140ºC<br />
Water solubility<br />
Practically insoluble<br />
Log octanol-water partition coefficient 3.12-3.2<br />
Vapor pressure at 25 ºC 7.99 mmHg at 25 ºC<br />
Figure D3-5<br />
Structure of Xylenes (from National Library of Medicine)***<br />
D3.1.2.1.2 Environmental Transformation and Degradation of Carriers and Dispersants<br />
1,2,4-TRIMETHYLBENZENE<br />
If released to the atmosphere, 1,2,4-trimethylbenzene will exist in the vapor phase, where it is<br />
subject to degradation by hydroxyl and nitrate radicals but not by direct photolysis (HSDB<br />
2009). 1,2,4-trimethylbenzene is expected to partition to organic matter if released to soil; and as<br />
a result, should have only limited mobility in this environmental <strong>com</strong>partment. Aerobic<br />
biodegradation may occur in soils. In surface waters, 1,2,4-trimethylbenzene will partition to<br />
sediment, although some is also expected to volatilize based on the Henry’s Law Constant.<br />
Hydrolysis is not a significant loss process (HSDB 2009).<br />
D3-16 App D_HHRA_508.doc JULY 2009