ether N D2O O Mg
ether N D2O O Mg
ether N D2O O Mg
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
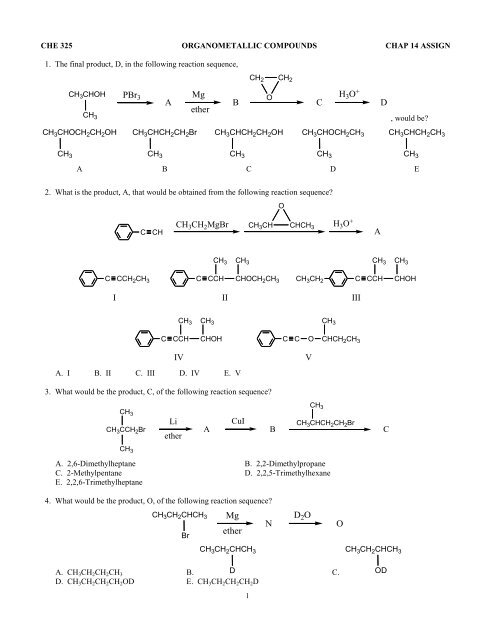
CHE 325 ORGANOMETALLIC COMPOUNDS CHAP 14 ASSIGN<br />
1. The final product, D, in the following reaction sequence,<br />
CH 2 CH 2<br />
CH 3 CHOH PBr 3<br />
<strong>Mg</strong><br />
O<br />
H 3 O +<br />
A<br />
B<br />
C<br />
D<br />
<strong>ether</strong><br />
CH 3<br />
, would be<br />
CH 3 CHOCH 2 CH 2 OH CH 3 CHCH 2 CH 2 Br CH 3 CHCH 2 CH 2 OH CH 3 CHOCH 2 CH 3 CH 3 CHCH 2 CH 3<br />
CH 3 CH 3 CH 3 CH 3 CH 3<br />
A B C D E<br />
2. What is the product, A, that would be obtained from the following reaction sequence<br />
O<br />
CH 3 CH 2 <strong>Mg</strong>Br CH 3 CH CHCH 3<br />
H 3 O +<br />
C CH A<br />
CH 3 CH 3<br />
CHOCH 2 CH 3<br />
C CCH<br />
CH 3<br />
CH 3<br />
C CCH 2 CH 3 C CCH<br />
CH 3 CH 2<br />
CHOH<br />
I II III<br />
CH 3<br />
CH 3<br />
C<br />
CCH<br />
CH 3<br />
C C CHCH 2 CH 3<br />
CHOH<br />
O<br />
IV<br />
A. I B. II C. III D. IV E. V<br />
V<br />
3. What would be the product, C, of the following reaction sequence<br />
CH 3<br />
CH 3 CCH 2 Br<br />
Li<br />
<strong>ether</strong><br />
A<br />
CuI<br />
B<br />
CH 3<br />
CH 3 CHCH 2 CH 2 Br<br />
C<br />
CH 3<br />
A. 2,6-Dimethylheptane B. 2,2-Dimethylpropane<br />
C. 2-Methylpentane D. 2,2,5-Trimethylhexane<br />
E. 2,2,6-Trimethylheptane<br />
4. What would be the product, O, of the following reaction sequence<br />
CH 3 CH 2 CHCH 3 <strong>Mg</strong><br />
N<br />
<strong>ether</strong><br />
Br<br />
D 2 O<br />
O<br />
CH 3 CH 2 CHCH 3<br />
A. CH 3 CH 2 CH 2 CH 3 B. D C.<br />
D. CH 3 CH 2 CH 2 CH 2 OD E. CH 3 CH 2 CH 2 CH 2 D<br />
1<br />
CH 3 CH 2 CHCH 3<br />
OD
5. What is the product, A, that would be obtained from the following reaction sequence<br />
A. I<br />
B. II<br />
C. III<br />
D. IV<br />
E. V<br />
OH<br />
OH<br />
PBr 3 Li (CH 3 ) 2 C=O NH 4 Cl<br />
OH<br />
10 o C<br />
A<br />
I<br />
II<br />
III<br />
HO<br />
IV<br />
V<br />
6. What would be the final product, A, in the following reaction sequence<br />
A. I<br />
B. II<br />
C. III<br />
D. IV<br />
E. V<br />
O<br />
OH<br />
O<br />
PBr 3 <strong>Mg</strong>, Et 2 O H 3 O +<br />
OH<br />
heat<br />
A<br />
OH<br />
I II III<br />
Br<br />
O<br />
OH<br />
IV<br />
V<br />
7. What would be the major product of the following reaction<br />
A. (R)-3-ethyl-5-methylheptane<br />
B. (R,S)-3-ethyl-5-methylheptane<br />
C. (S)-3-ethyl-5-methylheptane<br />
D. (3R,5S)-5-ethyl-3-methylheptane<br />
E. (3S,5R)-5-ethyl-3-methylheptane<br />
Cl<br />
Li, Et 2 O<br />
CuI<br />
H<br />
Br<br />
CH 3<br />
A<br />
8. What is the principal product(s) formed when 1 mol of methylmagnesium iodide reacts with 1 mol of<br />
p-hydroxyacetophenone <br />
A. I<br />
B. II<br />
C. III<br />
D. IV<br />
E. V<br />
I<strong>Mg</strong>O<br />
O<br />
HO<br />
CH 3 <strong>Mg</strong>I<br />
O<strong>Mg</strong>I<br />
+ CH 4 HO<br />
O<br />
I II III<br />
O<br />
O<br />
A<br />
O<br />
O<br />
O <strong>Mg</strong>I I<strong>Mg</strong>O<br />
IV<br />
V<br />
2
OH H<br />
9. What product(s) is/are produced in the 1:1 reaction of sec-butylmagnesium bromide with<br />
CH 3 CHCH 2 CH 2 C<br />
OH O<strong>Mg</strong>Br<br />
O<br />
<br />
CH 3 CHCH 2 CH 2 CHCCH 2 CH 3<br />
O<strong>Mg</strong>Br<br />
H<br />
A.<br />
CH 3 B.<br />
CH 3 CHCH 2 CH 2 C<br />
O + CH 3 CH 2 CH 2 CH 3<br />
OH<br />
O<strong>Mg</strong>Br<br />
O<strong>Mg</strong>Br<br />
H<br />
C. CH 3CHCH 2 CH 2 CHCH 2 CH(CH 3 ) 2<br />
H<br />
D. CH 3CHCH 2 CH 2 C<br />
O + (CH 3 ) 3 CH<br />
CH 3 CHCH 2 CH 2 C<br />
O<br />
OCHCH 2 CH 3<br />
E.<br />
CH 3<br />
10. What is the principal product of the following reaction:<br />
O<br />
CH 3 CH CH 2<br />
CH 3 CHCH 2 CH 2 CH 2 CH 3<br />
+ CH 3 CH 2 CH 2 CH 2 <strong>Mg</strong>Br/<strong>ether</strong>; then H 2 O <br />
OH<br />
A)<br />
CH 2 OH B. CH 3CHCH 2 CH 2 CH 2 CH 2 CH 3<br />
OH<br />
C. CH 3CHCH 2 OCH 2 CH 2 CH 2 CH 3<br />
D.<br />
CH 3 CHOCH 2 CH 2 CH 2 CH 3<br />
CH 2 OH<br />
CH 3 CHCH 2 OCH 2 CH 2 CH 2 CH 3<br />
E.<br />
OCH 2 CH 2 CH 2 CH 3<br />
11. The reaction of lithium diethylcuprate with 1-bromo-4,4-dimethylhexane yields:<br />
A. 3,3-Dimethylheptane B. 3,3-Dimethyloctane<br />
C. 1-Ethyl-4,4-dimethylhexane D. Di-4,4-dimethylhexylcuprate<br />
E. None of the above<br />
12. Which of the following synthetic procedures would be employed most effectively to transform ethanol into ethyl propyl<br />
<strong>ether</strong><br />
A. Ethanol + HBr, then <strong>Mg</strong>/<strong>ether</strong>, then H 3 O + , then NaH, then CH 3 CH 2 Br<br />
B. Ethanol + HBr, then <strong>Mg</strong>/<strong>ether</strong>, then HCHO, then H 3 O + , then NaH, then CH 3 CH 2 Br<br />
C. Ethanol + CH 3 CH 2 CH 2 OH + H 2 SO 4 /140C<br />
D. Ethanol + NaH, then HCHO, then H 3 O + , then HBr, then <strong>Mg</strong>/<strong>ether</strong>, then CH 3 CH 2 CH 2 Br<br />
E. Ethanol + H 2 SO 4 /180°C, then CH 3 CH 2 CH 2 Br<br />
3
13. What compound(s) result(s) from the reaction of CH 3 CH 2 CH 2 <strong>Mg</strong>Br with CH 3 CH 2 CH 2 CH 2 CO 2 H (1:1 mole ratio)<br />
(CH 3 CH 2 CH 2 ) 2 CCH 2 CH 2 CH 2 CH 3<br />
O<br />
A.<br />
OH B. CH 3CH 2 CH 2 CCH 2 CH 2 CH 2 CH 3<br />
O<br />
C. CH 3CH 2 CH 2 CH 2 COCH 2 CH 2 CH 3<br />
D. CH 3 CH 2 CH 3 + CH 3 CH 2 CH 2 CH 2 CO 2 <strong>Mg</strong>Br<br />
O<br />
O<br />
E. CH 3CH 2 COCCH 2 CH 2 CH 2 CH 3<br />
C 6 H 5<br />
CH 3 CH 2 CH 2 CCH 3<br />
14. Your task is to synthesize<br />
OH through a Grignard synthesis. Which pairs of compounds listed below<br />
would you choose as starting materials<br />
O<br />
O<br />
A.<br />
CH 3 CH 2 CH 2 Br and<br />
CH 3 CC 6 H 5<br />
B. CH 3CH 2 CH 2 CH and C 6 H 5 Br<br />
O<br />
C. C 6H 5 CH<br />
and<br />
CH 3 CH 2 CHCH 3<br />
Br<br />
D. More than one of these. Which ones<br />
E. None of these<br />
15. Your task is to synthesize 2-phenyl-2-hexanol through a Grignard synthesis. Which pair(s) of compounds listed below<br />
would you choose as starting materials<br />
A.<br />
CH 3 CH 2 CH 2 Br and<br />
O<br />
O<br />
CH 3 CC 6 H 5<br />
B.<br />
CH 3 CHCH 2 Br<br />
CH 3<br />
and<br />
O<br />
CH 3 CC 6 H 5<br />
C. CH 3CH 2 CH 2 CH 2 CCH 3 and C 6 H 5 Br<br />
D. Answers A or B<br />
E. Answers A or C<br />
16. Which combination of reagents is to be preferred for the synthesis of 2,4-dimethylhexane by the Corey-Posner,<br />
Whitesides-House procedure<br />
A. Lithium diisobutylcuprate + sec-butyl bromide<br />
B. Lithium dimethylcuprate + 2-bromo-4-methylhexane<br />
C. Lithium dimethylcuprate + 4-bromo-2-methylhexane<br />
D. Lithium diisopropylcuprate + 1-bromo-2-methylbutane<br />
E. Lithium di(2-methylbutyl)cuprate + isopropyl bromide<br />
4
17. Which of the following would serve as a synthesis of racemic 2-methyl-1-phenyl-2-butanol<br />
A. I<br />
B. II<br />
C. III<br />
D. All of the above<br />
E. None of the above<br />
CH 3<br />
OH<br />
<br />
CCH 2 CH 3<br />
CH 2 <strong>Mg</strong>Cl<br />
I<br />
CH 3 CH 2 CCH 3<br />
1. Et 2 O<br />
+<br />
O 2. NH<br />
+ 4<br />
II<br />
O<br />
CH 2 CCH 3<br />
+ CH 3 CH 2 <strong>Mg</strong>Br<br />
1. Et 2 O<br />
2. NH 4<br />
+<br />
III<br />
CH 2 CCH 2 CH 3<br />
O<br />
+ CH 3 <strong>Mg</strong>I<br />
1. Et 2 O<br />
2. NH 4<br />
+<br />
18. Which Grignard synthesis will produce an optically active product or product mixture<br />
<strong>Mg</strong>Br<br />
A.<br />
+<br />
O<br />
+ Cl<strong>Mg</strong><br />
O<br />
B.<br />
C.<br />
O<br />
O<br />
+<br />
<strong>Mg</strong>I<br />
D.<br />
O<br />
+<br />
H CH 3<br />
<strong>Mg</strong>I<br />
E. O<br />
H 3 C<br />
H<br />
+<br />
<strong>Mg</strong>Br<br />
19. Which of the following is the strongest acid<br />
A. R<strong>Mg</strong>X B. <strong>Mg</strong>(OH)X C. RH D. H 2 O<br />
20. Which of the following is the strongest base<br />
A. R<strong>Mg</strong>X B. <strong>Mg</strong>(OH)X C. RH D. H 2 O<br />
21. Grignard reagents react with oxirane (ethylene oxide) to form 1 alcohols but can be prepared in tetrahydrofuran solvent.<br />
Why is this difference in behavior observed<br />
A. Steric hindrance in the case of tetrahydrofuran precludes reaction with the Grignard.<br />
B. There is a better leaving group in the oxirane molecule.<br />
C. The oxirane ring is the more highly strained.<br />
D. It is easier to obtain tetrahydrofuran in anhydrous condition.<br />
E. Oxirane is a cyclic <strong>ether</strong>, while tetrahydrofuran is a hydrocarbon.<br />
22. Which of these compounds can be used to prepare the corresponding Grignard reagent<br />
A. CH 3 CHOHCH 2 CH 2 CH 2 CH 2 Br<br />
B. (CH 3 ) 3 CHCHBrCH 2 CH 2 CO 2 H<br />
C. BrCH=CHCH 2 CH 2 CH 3<br />
D. CH 3 NHCH 2 CH 2 Br<br />
E. None of the above can be used to prepare the corresponding Grignard reagent<br />
5
23. Which of these compounds cannot be used to prepare the corresponding Grignard reagent<br />
A. CH 3 OCH 2 CH 2 CH 2 Br B. (CH 3 ) 3 CCl<br />
C. CH 2 =CHCH 2 Br D. (CH 3 ) 2 NCH 2 CH 2 Br<br />
H<br />
E. O CCH 2 CH 2 I<br />
24. Which of these is the least reactive type of organometallic compound<br />
A. RK B. R 2 Hg C. RLi D. R 2 Zn E. R 3 Al<br />
25. If the role of the solvent is to assist in the preparation and stabilization of the Grignard reagent by coordination with the<br />
magnesium, which of these solvents should be least effective<br />
A. I<br />
B. II<br />
C. III<br />
D. IV<br />
E. V<br />
CH 3 CH 2 OCH 2 CH 3<br />
O<br />
(CH 3 CH 2 ) 3 N<br />
I II III<br />
CH 3 (CH 2 ) 4 CH 3 CH 3 OCH 2 CH 2 OCH 3<br />
IV<br />
V<br />
26. Which of these is most likely to be a successful synthesis of an organometallic compound<br />
A. CH 3 CH 2 CH 2 <strong>Mg</strong>Br + LiCl CH 3 CH 2 CH 2 Li + <strong>Mg</strong>BrCl<br />
B. 2 CH 3 CH 2 CH 2 CH 2 Li + ZnCl 2 (CH 3 CH 2 CH 2 CH 2 ) 2 Zn + 2 LiCl<br />
C. 3 (CH 3 CH 2 ) 2 Hg + 2 AlCl 3 2 (CH 3 CH 2 ) 3 Al + 3 HgCl 2<br />
D. (CH 3 CH 2 ) 3 Al + 3 NaCl 3 CH 3 CH 2 Na + AlCl 3<br />
E. (CH 3 ) 2 Cu + <strong>Mg</strong>Br 2 (CH 3 ) 2 <strong>Mg</strong> + CuBr 2<br />
27. What is the product of the following reaction<br />
1) HO<br />
OH 3)<br />
O<br />
OH<br />
OH<br />
O<br />
1) 2 CH 3 <strong>Mg</strong>Br<br />
2) H 3 O +<br />
2) OH 4)<br />
OH<br />
28. 1-Phenylnaphthalene, shown below, can be prepared in over 80% yield by one of the reactions below. Which one<br />
Br<br />
C 6 H 6 , AlCl 3<br />
1) 3)<br />
I<br />
(C 6 H 5 ) 2 CuLi<br />
2) 4)<br />
diethyl <strong>ether</strong><br />
Cl<br />
C 6 H 5 <strong>Mg</strong>Br<br />
diethyl <strong>ether</strong><br />
C 6 H 5 Br, AlCl 3<br />
C 6 H 5<br />
6
NAME_______________________________________<br />
DATE______________<br />
ANSWER SHEET<br />
CHE 325 – CHAP 14 ASSIGN<br />
1. _______________ 13. _______________ 25. _______________ 37. _______________<br />
2. _______________ 14. _______________ 26. _______________ 38. _______________<br />
3. _______________ 15. _______________ 27. _______________ 39. _______________<br />
4. _______________ 16. _______________ 28. _______________ 40. _______________<br />
5. _______________ 17. _______________ 29. _______________ 41. _______________<br />
6. _______________ 18. _______________ 30. _______________ 42. _______________<br />
7. _______________ 19. _______________ 31. _______________ 43. _______________<br />
8. _______________ 20. _______________ 32. _______________ 44. _______________<br />
9. _______________ 21. _______________ 33. _______________ 45. _______________<br />
10. _______________ 22. _______________ 34. _______________ 46. _______________<br />
11. _______________ 23. _______________ 35. _______________ 47. _______________<br />
12. _______________ 24. _______________ 36. _______________ 48. _______________<br />
SS II 2013<br />
7