CHE 275 STRUCTURE DETERMINES PROPERTIES CHAP 1 ...
CHE 275 STRUCTURE DETERMINES PROPERTIES CHAP 1 ...
CHE 275 STRUCTURE DETERMINES PROPERTIES CHAP 1 ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
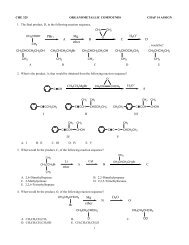
<strong>CHE</strong> <strong>275</strong> <strong>STRUCTURE</strong> <strong>DETERMINES</strong> <strong>PROPERTIES</strong> <strong>CHAP</strong> 1 ASSIGN1. The oxygen atom of a ketone (such as cyclohexanone) contains 2 lone pairs of electrons. These pairs of electrons mostlikely reside in what type of orbital?A. s orbitalB. p orbitalC. sp 3 orbitalD. sp 2 orbitalE. sp orbital2. Which electron dot structure correctly represents formaldehyde, CH 2 O?A. B. C. D. E.3. Which of the following structures has carbon atoms which are all sp 3 hybridized?A B C D E4. What bond angles would you expect to have around the indicated carbon?A. 130°B. 120°C. 109°D. 90°E. 60°5. Welwistatin (shown below) is a structurally interesting, newly discovered cytotoxic agent. How many hydrogen atoms arecontained in welwistatin?A. 20 hydrogen atomsB. 19 hydrogen atomsC. 18 hydrogen atomsD. 13 hydrogen atomsE. 10 hydrogen atoms6. An sp 3 hybridized orbital has what percentage s character?A. 0% s character B. 25% s characterC. 33.33% s character D. 50% s characterE. 100% s character1
7. The condensed formula (CH 3 ) 3 CCH 2 CH(OH)C 6 H 5 is represented by which of the following bond-line structures?A B C D E8. Which of the following structures is correctly represented by the molecular formula: C 8 H 10 O 2 ?A. B.C. D.E.9. The compound shown below [2-(4'-methoxyphenyl)-3-(3",4",5"-trimethoxybenzoyl)-6-methoxybenzo[b]thiophene] is anovel chemotherapeutic agent which functions by inhibiting tubulin polymerization. How many carbon atoms are sp 2hybridized in this molecule?A. No carbon atoms are sp 2 hybridized in this molecule.B. Twenty-one carbon atoms are sp 2 hybridized.C. Two carbon atoms are sp 2 hybridized.D. Eighteen carbon atoms are sp 2 hybridized.E. Twenty carbon atoms are sp 2 hybridized.10. How many carbon atoms are sp hybridized in the following molecule?A. 0 carbon atoms are sp hybridized.B. 2 carbon atoms are sp hybridized.C. 3 carbon atoms are sp hybridized.D. 6 carbon atoms are sp hybridized.E. 11 carbon atoms are sp hybridized.11. What geometry does the indicated carbon exhibit?A. pyramidalB. linearC. trigonal planarD. tetrahedralE. square planar2
12. Based on your knowledge of bonding and hybridization, which of the following statements is true for the carbon atomindicated by the arrow in the following structure?A. The carbon atom is sp 2 hybridized.B. The carbon atom is sp 3 hybridized.C. The carbon atom is sp hybridized.D. This carbon atom is hypervalent (contains 5 bonds or more) and the structure itself is not valid.E. This carbon atom is only able to bond through pi bond interactions. No sigma bonds are involved.13. Ozone (O 3 ) receives considerable attention due in part to our environmental interests associated with the Earth's ozonelayer. Choose the Lewis structure which best represents O 3 .A. B.C. D.E.14. Select the pair of structures which represent correctly drawn contributing resonance forms.A. B.C. D.E.15. With regard to resonance structures, which of the following statements is true?A. Resonance structures undergo rapid interconversion.B. No more than one atom may change connectivity between valid resonance structures.C. In determining resonance structures, only electrons and charge may change location.D. Resonance structures impart instability to a molecule.E. Only two resonance forms may exist for any one molecule.3
16. The condensed structure shown below is correctly represented by which of the following bond-line formulas (also calledzig-zag structures)?A. B.C. D.E.17. Select a structure which contains four sp 2 hybridized carbon atoms.A B C D E18. Which hydrogen in the following molecule would you expect to be the most acidic (i.e., most easily removed as H + )?A. B.C. D.E. None of these can be considered acidic.19. What is the correct descending order of electronegativity of the following elements?A. F > N > O > C > Na B. F > C > O > N > Na C. F > O > N > C > Na D. F > O > N > Na > C20. What is the charge (from left to right) on each of the species below?A. +1, −1, −1, 0 B. −1, 0, +1, 0 C. 0, +1, +1, 0 D. +1, 0, +1, 021. Which pair of elements would form an ionic bond?A. H and F B. H and C C. S and N D. Li and F4
22. For each of the following three bonds, specify whether the negative end of the dipole is towards the left or the right.C—Br Cl—H H—HA B CA. A-left, B-left, C-no dipole B. A-left, B-right, C-no dipoleC. A-right, B-left, C-no dipole D. one cannot decide unless all other attached elements are given23. The correct electronic configuration of nitrogen isA. 1s 2 2s 2 2p x 2 2p y1B. 1s 2 2s 1 2p x 1 2p y 1 2p z1C. 1s 2 2s 2 2p x 1 2p y 1 2p z1D. 1s 2 2s 2 2p x 2 2p y 1 2p z124. Listed below are the electron-dot formulas for several simple molecules and ions, including formal charges wherenecessary. Which of them are correct Lewis structures?A. A, B B. A, B, D C. B, C D. A, D25. Which structure and formal charge distribution is correct for diazaomethane, CH 2 N 2 ?A B C D26. Which of the following species have no formal charge on their carbon atom?A. A, C B. A, D C. B, C D. B, D27. Which of the following structures have one or more atoms with trigonal geometry? (charges have been omitted)H 3 C: H 4 N H 2 C=CH 2 BF 3A B C DA. A, C B. B, D C. A, C, D D. C, D28. Which of the following molecules would have a dipole moment?CH 2 Cl 2 CCl 4 CH 3 Cl H 2 C=CH 2 H-CC-H H 3 O+A B C D E FA. all but B B. A, C, and F C. C only D. C and F29. The formal charges on nitrogen and the starred oxygen atom of the compound show below areA. N +1; O* 0B. N +1; O* -1C. N +1; O* +1D. N -1; O* 05
30. Which of the following species have tetrahedral bond angles?NH 3 BF 3+CH 3−CH 3 H 2 O H 3 O +A B C D E FA. A, D and E B. A, E C. A, D, E, F D. only D31. Which of the following species is planar?CH 3+BF 3 H 3 O + +NH 4 H 2 C=CH 2 AlCl 3A B C D E FA. all but C and D B. A and B C. all but C D. B, E, F32. How many constitutional isomers are possible for C 4 H 10 O?A. three B. four C. five D. seven33. In the reaction below, the strongest base isA H 2 O B. NH 2‒C. HO ‒ D. NH 3H 2 O +NH 2‒ HO ‒ + NH 334. A thorough study of organic chemistry requires a basic understanding of acid and base strengths. Which of the followingmolecules most likely has a pKa = 15.5?A. H 2 SO 4 B. HCl C. Methanol D. Methane E. Acetic Acid35. Match the hybridization of carbon with the correct electron domain shapeA. sp 3 X. trigonalB. sp 2 Y. linearC. sp Z. tetrahedralA. AX, BY, CZ B. AZ, BX, CY C. AY, BZ, CX D. AX, BZ, CY36. Which is the shortest of the carbon-carbon single bonds indicated by arrows in the following compounds?CH 2 H 2 CH 3 C CH 3 H 3 C C CH H 3 C CH HC C C CH HC C CHA B C D E37. Taxol (see structure below) is a potent chemotherapeutic agent (isolated from the Pacific Yew tree) which is especiallyeffective against ovarian cancer. Which of the following functional groups is not contained in taxol?A. KetoneB. EsterC. AmideD. AmineE. Alcohol6
38. For the simple hydrides, MH n , acidities increase in the order:A. CH 4 , NH 3 , H 2 O , H 2 S , HBr B. HBr , H 2 S , H 2 O , NH 3 , CH 4C. HBr , H 2 O , NH 3 , H 2 S , CH 4 D. NH 3 , H 2 S , CH 4 , H 2 O , HBrE. H 2 S , H 2 O , HBr , NH 3 , CH 439. Which acid-base reaction would not take place as written?A. CH 3 Li + CH 3 CH 2 CH 2 CH 2 NH 2 CH 4 + CH 3 CH 2 CH 2 CH 2 NHLiB. CH 3 CCH + NaOCH 3 CH 3 CCNa + CH 3 OHC. HCCNa + H 2 O HCCH + NaOHD. CH 3 OH + NaNH 2 CH 3 ONa + NH 3E. CH 3 CO 2 H + CH 3 ONa CH 3 CO 2 Na + CH 3 OH40. A group of acids arranged in order of decreasing acidity is:HNO 3 > CH 3 COOH > C 6 H 5 OH > H 2 O > HCCHWhat is the arrangement of the conjugate bases of these compounds in decreasing order of basicity?A. NO 3 > CH 3 COO > C 6 H 5 O > OH > HCC B. CH 3 COO > C 6 H 5 O > NO 3 > OH > HCC C. C 6 H 5 O > NO 3 > HCC > OH > CH 3 COO D. HCC > OH > C 6 H 5 O > CH 3 COO > NO 3E. No prediction of relative base strength is possible.NAME__________________________________________DATE___________________ANSWER SHEET<strong>CHE</strong> <strong>275</strong> – <strong>CHAP</strong> 1 ASSIGN1. _________________ 11. _________________ 21. _________________ 31. _________________2. _________________ 12. _________________ 22. _________________ 32. _________________3. _________________ 13. _________________ 23. _________________ 33. _________________4. _________________ 14. _________________ 24. _________________ 34. _________________5. _________________ 15. _________________ 25. _________________ 35. _________________6. _________________ 16. _________________ 26. _________________ 36. _________________7. _________________ 17. _________________ 27. _________________ 37. _________________8. _________________ 18. _________________ 28. _________________ 38. _________________9. _________________ 19. _________________ 29. _________________ 39. _________________10. _________________ 20. _________________ 30. _________________ 40. _________________SS I 20137